Wnt-5a-ROR2信号在肾细胞癌中引发转移性定植和血管生成,梅花素抑制了该轴的激活。
IF 3.6
2区 医学
Q1 PATHOLOGY
引用次数: 0
摘要
Wnt-5a 是一种由 WNT5A 基因编码的蛋白质,是 ROR2 受体的配体。然而,它对透明细胞肾细胞癌(ccRCC)的生物学影响仍不清楚。本研究观察了WNT5A和ROR2同时表达水平对预测不利总生存期和疾病特异性生存期的预后意义。在ccRCC细胞系中检测到了Wnt-5a的高表达,而在正常近端肾小管细胞系HK-2细胞中却没有检测到。用5-azaC抑制786-O和Caki-2细胞中的DNA甲基转移酶会导致Wnt-5a上调,这表明可能存在表观遗传修饰。此外,研究结果表明,敲除 WNT5A 和 ROR2 后,体外细胞移动和体内转移定植均受到抑制。沉默 WNT5A 和 ROR2 表达后,体内血管生成和体外内皮细胞管状结构形成也受到抑制。此外,还发现 Wnt-5a-ROR2 信号转导下游基因特征的改变与 MTA1-CTNNB1 轴相似。此外,研究还发现普鲁尼丁能逆转 Wnt-5a-ROR2 信号激活所产生的基因特征,并能抑制 ccRCC 细胞的迁移和增殖。总之,这项研究证明了Wnt-5a-ROR2轴的临床和功能意义,并确定了普鲁尼丁是治疗携带异常Wnt-5a-ROR2信号通路的ccRCC患者的潜在精准药物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
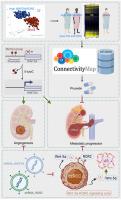
Wnt-5a–Receptor Tyrosine Kinase-Like Orphan Receptor 2 Signaling Provokes Metastatic Colonization and Angiogenesis in Renal Cell Carcinoma, and Prunetin Supresses the Axis Activation
Wnt-5a is a protein encoded by the WNT5A gene and is a ligand for the receptor tyrosine kinase-like orphan receptor 2 (ROR2). However, its biological impact on clear cell renal cell carcinoma (ccRCC) remains unclear. In this study, the prognostic significance of concurrent WNT5A and ROR2 expression levels was observed to predict unfavorable overall survival and disease-specific survival. High Wnt-5a expression was detected in a ccRCC cell line panel but not in HK-2 cells, a normal proximal tubular cell line. Inhibition of DNA methyltransferase by 5-azacytidine in 786-O and Caki-2 cells resulted in Wnt-5a up-regulation, indicating potential epigenetic modification. Furthermore, there was a repression of cell movement in vitro and metastatic colonization in vivo on WNT5A and ROR2 knockdown. Suppressions of angiogenesis in vivo and tubular-like structure formation in endothelial cells in vitro were also observed after silencing WNT5A and ROR2 expression. In addition, alteration in the downstream gene signature of the Wnt-5a–ROR2 signaling was similar to that in metastasis-associated gene 1–β-catenin axis. Moreover, prunetin treatment reversed the gene signature derived from Wnt-5a–ROR2 signaling activation and to abolish ccRCC cell migration and proliferation. Overall, this study demonstrates the clinical and functional significance of the Wnt-5a–ROR2 axis and identifies prunetin as a potential precision medicine for patients with ccRCC harboring aberrant Wnt-5a–ROR2 signaling pathways.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.40
自引率
0.00%
发文量
178
审稿时长
30 days
期刊介绍:
The American Journal of Pathology, official journal of the American Society for Investigative Pathology, published by Elsevier, Inc., seeks high-quality original research reports, reviews, and commentaries related to the molecular and cellular basis of disease. The editors will consider basic, translational, and clinical investigations that directly address mechanisms of pathogenesis or provide a foundation for future mechanistic inquiries. Examples of such foundational investigations include data mining, identification of biomarkers, molecular pathology, and discovery research. Foundational studies that incorporate deep learning and artificial intelligence are also welcome. High priority is given to studies of human disease and relevant experimental models using molecular, cellular, and organismal approaches.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


