Acyl-CoA Synthetase Medium Chain Family Member 5 介导的脂肪酸代谢失调促进肝细胞癌的恶化
IF 4.7
2区 医学
Q1 PATHOLOGY
引用次数: 0
摘要
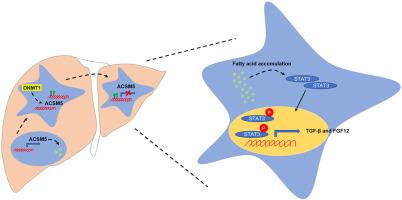
肝细胞癌(HCC)是最常见的原发性肝癌,在全世界的发病率和死亡率都很高。尽管在诊断和治疗方面取得了进展,但 HCC 对治疗的反应仍然不佳,预后较差。了解驱动 HCC 的分子机制对于开发有效疗法至关重要。新的证据表明,脂肪酸代谢失调是导致 HCC 的原因之一。参与脂肪酸代谢的酰基-CoA 中链合成酶 5(ACSM5)在 HCC 中被下调,但其作用还不十分清楚。在本研究中,我们分析了 ACSM5 在 HCC 患者样本和细胞系中的表达。利用新建立的ACSM5表达的HCC细胞系Huh7-ACSM5和Hepa1-6-ACSM5,我们研究了ACSM5的作用和调控机制。结果表明,与非肿瘤组织相比,ACSM5在HCC肿瘤组织中明显下调。ACSM5的表达受DNA甲基化的调控,DNMT1抑制剂能有效增加ACSM5的表达并降低启动子区的甲基化。在Huh7细胞中过表达ACSM5可减少脂肪酸积累,降低体外细胞增殖、迁移和侵袭,并抑制小鼠异种移植的肿瘤生长。此外,ACSM5 的过表达还降低了 STAT3 的磷酸化,进而影响下游细胞因子 TGFB 和 FGF12 的 mRNA 水平。我们的研究结果表明,ACSM5的下调有助于HCC的进展,这为我们深入了解其致癌作用提供了线索,并凸显了其作为HCC生物标记物和治疗靶点的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Acyl-CoA Synthetase Medium-Chain Family Member 5–Mediated Fatty Acid Metabolism Dysregulation Promotes the Progression of Hepatocellular Carcinoma
Hepatocellular carcinoma (HCC) is the most common primary liver cancer, with high incidence and mortality worldwide. Despite diagnostic and therapeutic advancements, HCC remains poorly responsive to treatment, with a poor prognosis. Understanding the molecular mechanisms driving HCC is crucial for developing effective therapies. Emerging evidence indicates that dysregulated fatty acid metabolism contributes to HCC. Acyl-CoA medium-chain synthetase 5 (ACSM5), involved in fatty acid metabolism, is down-regulated in HCC; however, its role is not well understood. This study was used to analyze ACSM5 expression in HCC patient samples and cell lines. The newly established ACSM5-overexpressing HCC cell lines, Huh7-ACSM5 and Hepa1-6–ACSM5, were used to investigate the effects and regulatory mechanisms of ACSM5. The results showed that ACSM5 was significantly down-regulated in HCC tumor tissues compared with non-tumor tissues. ACSM5 expression was regulated by DNA methylation, with a DNA methyltransferase 1 (DNMT1) inhibitor effectively increasing ACSM5 expression and reducing promoter region methylation. Overexpression of ACSM5 in Huh7 cells reduced fatty acid accumulation, decreased cell proliferation, migration, and invasion in vitro, and inhibited tumor growth in mouse xenografts. Furthermore, ACSM5 overexpression also decreased STAT3 phosphorylation, subsequently affecting downstream cytokine TGFB and FGF12 mRNA levels. These findings suggest that ACSM5 down-regulation contributes to HCC progression, providing insights into its oncogenic role and highlighting its potential as a biomarker and therapeutic target for HCC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.40
自引率
0.00%
发文量
178
审稿时长
30 days
期刊介绍:
The American Journal of Pathology, official journal of the American Society for Investigative Pathology, published by Elsevier, Inc., seeks high-quality original research reports, reviews, and commentaries related to the molecular and cellular basis of disease. The editors will consider basic, translational, and clinical investigations that directly address mechanisms of pathogenesis or provide a foundation for future mechanistic inquiries. Examples of such foundational investigations include data mining, identification of biomarkers, molecular pathology, and discovery research. Foundational studies that incorporate deep learning and artificial intelligence are also welcome. High priority is given to studies of human disease and relevant experimental models using molecular, cellular, and organismal approaches.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


