用 AAV1 和 AAV6 改善 G551D-CF 雪貂的气道和消化道疾病
IF 4.5
3区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
CF的基因治疗主要针对肺部。在这里,我们采用了一种不同的方法,将含有Δ27-264-CFTR(CFTR的截短版本)的AAV1或AAV6注入G551D CF雪貂的头静脉并喷入气管。为了诱导疾病表型,在灌注前停止使用增效剂 VX-770 治疗 7 天。事实上,所有雪貂在进入研究时都没有胰腺功能。四只雪貂(三只接受了 AAV1,一只接受了 AAV6)在载体输送 48 天后进行了尸检,四只(三只接受了 AAV6,一只接受了 AAV1)在计划尸检前安乐死或死亡。在所有气管和肺部样本以及接受治疗的雪貂的肝脏、胰腺和回肠中都检测到了 AAV1 或 AAV6 载体基因组、mRNA 表达和 CFTR 蛋白。气道表面和基底细胞、胰腺和胆管以及回肠隐窝和绒毛都被成功转导。在 48 天死亡的转导雪貂中发现,未经处理的雪貂呼吸道阻塞并伴有肺出血、胰腺和胆管堵塞以及回肠粘液栓塞。对 G551D 雪貂的转导表明,全身应用和气道应用相结合可能是治疗 CF 的首选途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
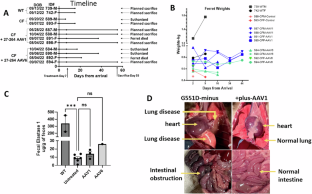
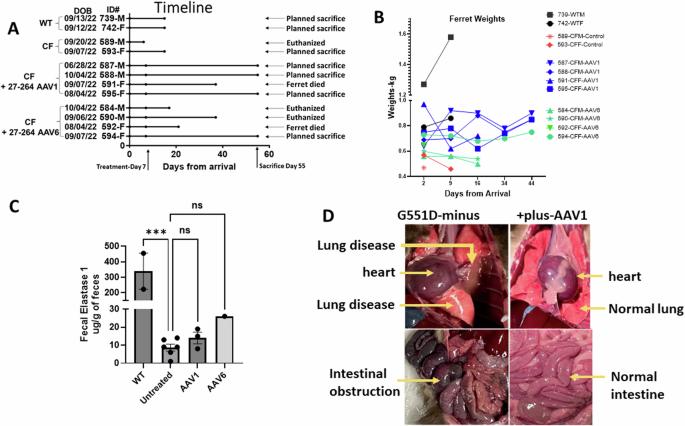
Amelioration of airway and GI disease in G551D-CF ferrets by AAV1 and AAV6
Gene therapy for CF has concentrated on targeting the lung. Here we took a different approach by injecting into the cephalic vein and spraying into the trachea of G551D, CF ferrets either AAV1 or 6 containing Δ27-264-CFTR, a truncated version of CFTR. Treatment with the potentiator VX-770 was halted for 7 days before instillation to induce a disease phenotype. Indeed, all ferrets were pancreas-insufficient when they entered the study. Four ferrets (three receiving AAV1 and one AAV6) were necropsied 48 days after vector delivery, and four (three receiving AAV6, one AAV1) were euthanized or died prior to the planned necropsy. AAV1 or AAV6 vector genomes, mRNA expression, and CFTR protein were detected in all tracheal and lung samples and in the liver, pancreas, and ileum of the treated ferrets. Surface and basal airway cells, pancreatic and bile ducts, and ileal crypts and villi were successfully transduced. Obstruction of the airways accompanied by pulmonary hemorrhaging, plugged pancreatic and bile ducts as well as mucous plugs in the ileum were noticed in untreated but absent from transduced ferrets necropsied at 48 days. Transduction of G551D ferrets suggests that a combination of systemic and airway application may be the preferred route of delivery for CF.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Gene Therapy
医学-生化与分子生物学
CiteScore
9.70
自引率
2.00%
发文量
67
审稿时长
4-8 weeks
期刊介绍:
Gene Therapy covers both the research and clinical applications of novel therapeutic techniques based on a genetic component. Over the last few decades, significant advances in technologies ranging from identifying novel genetic targets that cause disease through to clinical studies, which show therapeutic benefit, have elevated this multidisciplinary field to the forefront of modern medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


