溶瘤病毒疗法联合抗PD-L1抗体对结直肠癌患者的长期疗效及机制研究
IF 4.8
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
摘要
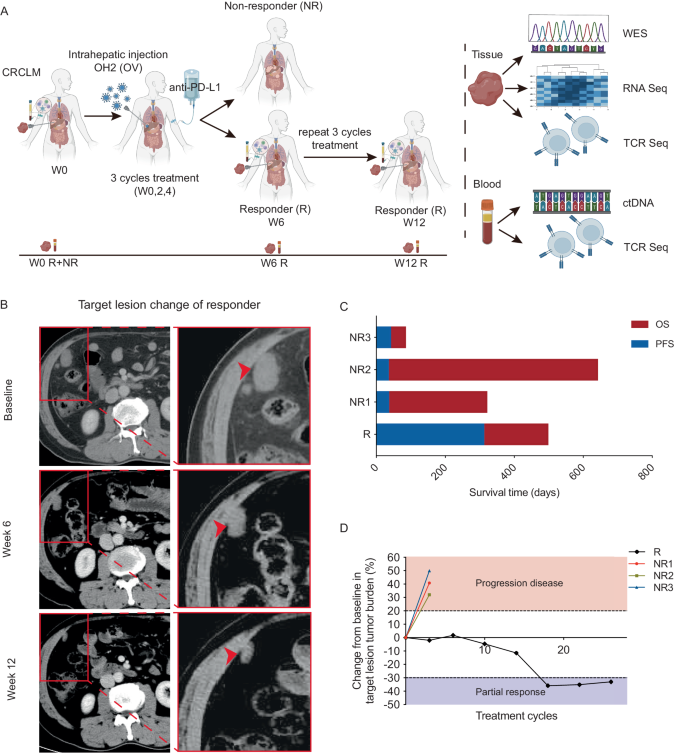
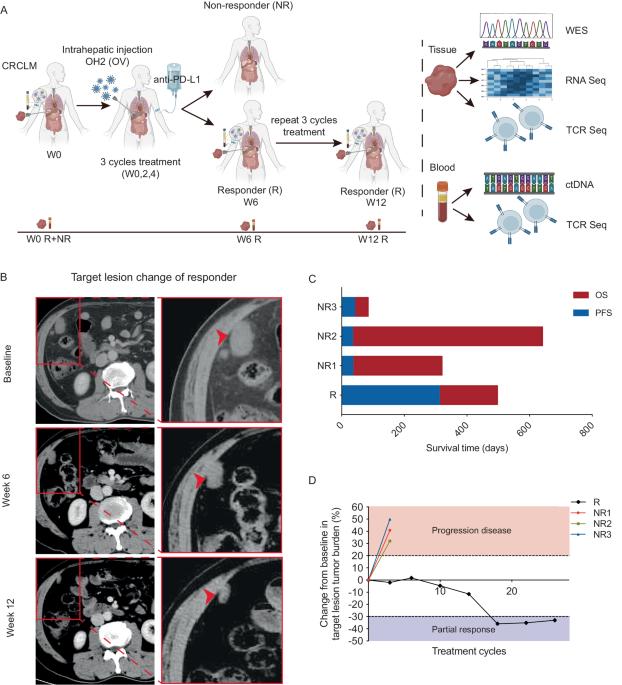
众所周知,结直肠癌(CRC)对免疫疗法具有抗药性。在我们的 I 期临床试验中,一名患者在溶瘤病毒疗法和免疫疗法的联合治疗中获得了 313 天的长期应答。为了深入了解潜在的分子机制,我们对这名患者和三名无应答者进行了全面的多组学分析。我们的研究发现,与非应答者相比,该应答者的肿瘤微环境(TME)最初只有极少量的T细胞和自然杀伤细胞浸润,同时巨噬细胞的存在也相对较多。值得注意的是,在治疗过程中,应答者肿瘤组织中的 CD4+ T 细胞、CD8+ T 细胞和 B 细胞逐渐增多。与此同时,与 T 细胞活化和细胞毒性相关的转录因子(包括 GATA3、EOMES 和 RUNX3)也显著上调。此外,对应答者外周血样本的动态监测显示,循环肿瘤DNA(ctDNA)迅速下降,表明其有可能成为治疗效果的早期血液生物标志物。总之,我们的研究结果证明了溶瘤病毒疗法和免疫疗法联合治疗某些 CRC 患者的有效性,并提供了分子证据,证明溶瘤病毒疗法有可能将 "冷 "TME 转变为 "热 "TME,从而提高免疫疗法的敏感性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
The long-term effectiveness and mechanism of oncolytic virotherapy combined with anti-PD-L1 antibody in colorectal cancer patient
Colorectal cancer (CRC) is known to be resistant to immunotherapy. In our phase-I clinical trial, one patient achieved a 313-day prolonged response during the combined treatment of oncolytic virotherapy and immunotherapy. To gain a deeper understanding of the potential molecular mechanisms, we performed a comprehensive multi-omics analysis on this patient and three non-responders. Our investigation unveiled that, initially, the tumor microenvironment (TME) of this responder presented minimal infiltration of T cells and natural killer cells, along with a relatively higher presence of macrophages compared to non-responders. Remarkably, during treatment, there was a progressive increase in CD4+ T cells, CD8+ T cells, and B cells in the responder’s tumor tissue. This was accompanied by a significant upregulation of transcription factors associated with T-cell activation and cytotoxicity, including GATA3, EOMES, and RUNX3. Furthermore, dynamic monitoring of peripheral blood samples from the responder revealed a rapid decrease in circulating tumor DNA (ctDNA), suggesting its potential as an early blood biomarker of treatment efficacy. Collectively, our findings demonstrate the effectiveness of combined oncolytic virotherapy and immunotherapy in certain CRC patients and provide molecular evidence that virotherapy can potentially transform a “cold” TME into a “hot” one, thereby improving sensitivity to immunotherapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


