通过协同质子耦合电子传递改进硝酸-氨电催化
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
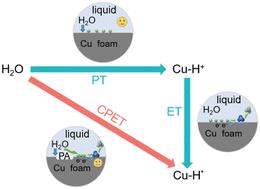
我们利用植酸盐在铜表面的配位,通过质子耦合电子传递途径克服了质子吸附/活化的动力学瓶颈;这使得硝酸-氨电催化的活性提高了一个数量级。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Improved nitrate-to-ammonia electrocatalysis through concerted proton-coupled electron transfer†
We used phytate coordination on a Cu surface to overcome the kinetic bottleneck for proton adsorption/activation through a concerted proton-coupled electron transfer pathway; this leads to one order of magnitude activity enhancement for nitrate-to-ammonia electrocatalysis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


