GATA2 通过抑制 IFN-β 轴介导的抗肿瘤免疫,促进耐阉割前列腺癌的发展
IF 6.9
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
长期接受雄激素剥夺疗法(ADT)治疗后,几乎不可避免地会患上阉割抵抗性前列腺癌(CRPC),从而导致大量死亡。研究驱动 CRPC 发展的机制势在必行。在这里,我们确定了在 CRPC 患者中经常扩增的先驱转录因子 GATA2 会抑制干扰素 (IFN)-β 介导的抗肿瘤免疫,从而促进 CRPC 的进展。我们利用基因工程小鼠模型(GEMM)证明,GATA2 的过表达会阻碍阉割诱导的细胞凋亡和肿瘤缩小,从而促进肿瘤转移和 CRPC 的发展。值得注意的是,GATA2 主要通过抑制阉割诱导的 IFN-β 信号激活和 CD8+ T 细胞浸润来驱动阉割抗性。这一发现与 CRPC 患者中 GATA2 表达和 IFNB1 表达以及 CD8+ T 细胞浸润之间的负相关性相吻合。从机理上讲,GATA2 招募了 PIAS1 作为核心抑制因子,并以雄激素依赖性的方式重编程了 IFN-β 轴的关键转录因子 IRF3 的表链。此外,我们还发现了一个新的沉默子元件,它通过与 IFNB1 启动子的循环促进了 GATA2 和 PIAS1 的功能。重要的是,删除 GATA2 可增强抗肿瘤免疫力并减轻 CRPC 的发展。因此,我们的研究结果阐明了一种新的机制,即 GATA2 通过抑制 IFN-β 轴介导的抗肿瘤免疫来促进 CRPC 的发展,从而突出了 GATA2 作为 CRPC 治疗靶点的前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
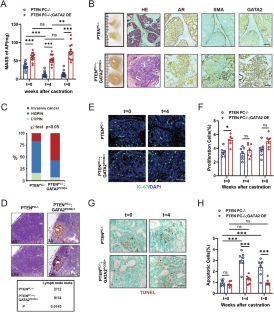
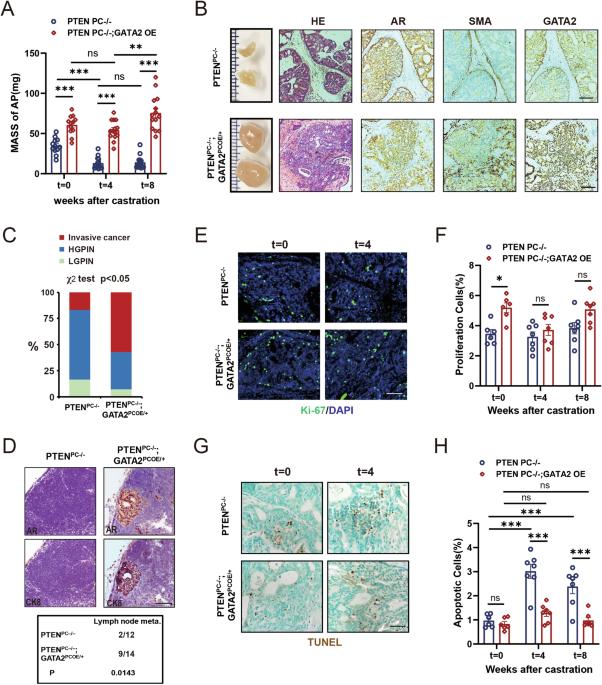
GATA2 promotes castration-resistant prostate cancer development by suppressing IFN-β axis-mediated antitumor immunity
Castration-resistant prostate cancer (CRPC) nearly inevitably develops after long-term treatment with androgen deprivation therapy (ADT), leading to significant mortality. Investigating the mechanisms driving CRPC development is imperative. Here, we determined that the pioneer transcription factor GATA2, which is frequently amplified in CRPC patients, inhibits interferon (IFN)-β-mediated antitumor immunity, thereby promoting CRPC progression. Employing a genetically engineered mouse model (GEMM), we demonstrated that GATA2 overexpression hindered castration-induced cell apoptosis and tumor shrinkage, facilitating tumor metastasis and CRPC development. Notably, GATA2 drives castration resistance predominantly via repressing castration-induced activation of IFN-β signaling and CD8+ T-cell infiltration. This finding aligns with the negative correlation between GATA2 expression and IFNB1 expression, as well as CD8+ T-cell infiltration in CRPC patients. Mechanistically, GATA2 recruited PIAS1 as corepressor, and reprogramed the cistrome of IRF3, a key transcription factor of the IFN-β axis, in an androgen-independent manner. Furthermore, we identified a novel silencer element that facilitated the function of GATA2 and PIAS1 through looping to the IFNB1 promoter. Importantly, depletion of GATA2 augmented antitumor immunity and attenuated CRPC development. Consequently, our findings elucidate a novel mechanism wherein GATA2 promotes CRPC progression by suppressing IFN-β axis-mediated antitumor immunity, underscoring GATA2 as a promising therapeutic target for CRPC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


