在骨髓增生异常肿瘤中,m6A甲基转移酶METTL14通过SETBP1介导的PI3K-AKT信号通路激活促进细胞增殖。
IF 12.8
1区 医学
Q1 HEMATOLOGY
引用次数: 0
摘要
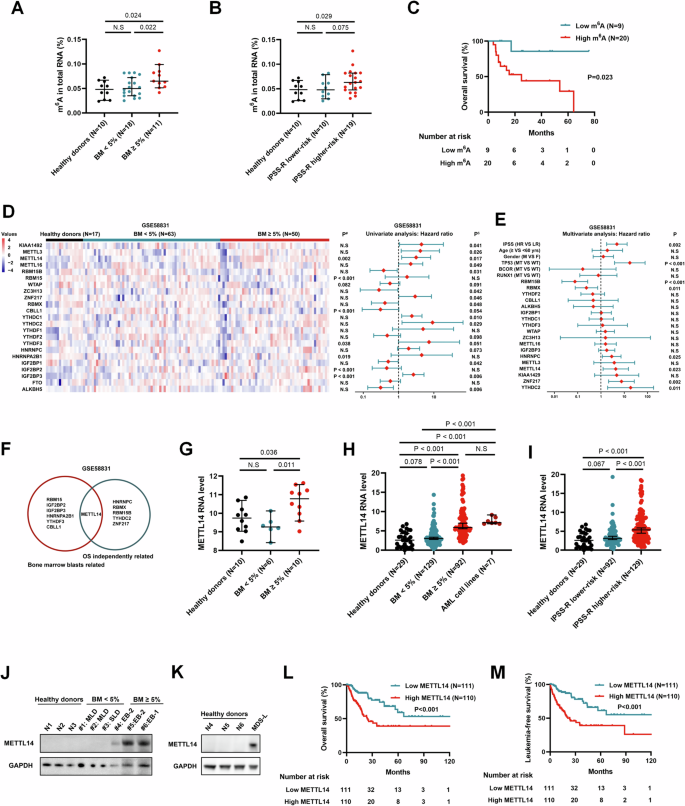
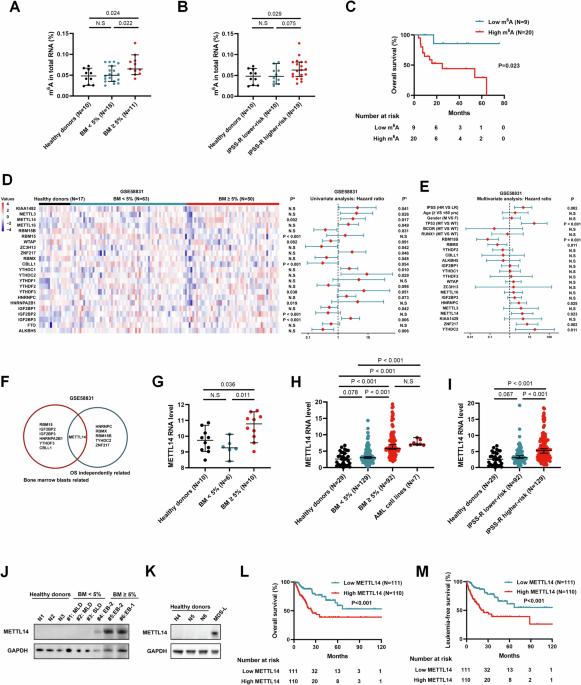
N6-甲基腺苷(m6A)是哺乳动物 mRNA 中最常见的表转录组修饰。最近的研究发现,m6A 与包括血液肿瘤在内的多种恶性肿瘤的发病机制有关。然而,m6A修饰和m6A调节因子在骨髓增生异常性肿瘤(MDS)中的具体作用仍鲜为人知。在本文中,我们证实骨髓细胞凋亡率≥5%的MDS患者的m6A水平和m6A甲基转移酶METTL14的表达均升高。此外,随着疾病风险的增加,m6A水平和METTL14的表达也随之上调,并与不良临床结果显著相关。敲除 METTL14 可抑制 MDS 细胞的增殖和集落形成能力。此外,体内实验显示,敲除METTL14能显著减轻肿瘤负荷,延长小鼠存活时间。从机理上讲,METTL14通过形成METTL3-METTL14复合物促进了SETBP1 mRNA的m6A修饰,从而增加了SETBP1 mRNA的稳定性,随后激活了PI3K-AKT信号通路。总之,这项研究阐明了METTL14/m6A/SETBP1/PI3K-AKT信号轴在MDS中的参与,凸显了靶向METTL3-METTL14复合物介导的m6A修饰治疗MDS的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
The m6A methyltransferase METTL14 promotes cell proliferation via SETBP1-mediated activation of PI3K-AKT signaling pathway in myelodysplastic neoplasms
N6-methyladenosine (m6A) is the most prevalent epitranscriptomic modification in mammalian mRNA. Recent studies have revealed m6A is involved in the pathogenesis of various malignant tumors including hematologic neoplasms. Nevertheless, the specific roles of m6A modification and m6A regulators in myelodysplastic neoplasms (MDS) remain poorly understood. Herein, we demonstrated that m6A level and the expression of m6A methyltransferase METTL14 were elevated in MDS patients with bone marrow blasts ≥5%. Additionally, m6A level and METTL14 expression were upregulated as the disease risk increased and significantly associated with adverse clinical outcomes. Knockdown of METTL14 inhibited cell proliferation and colony formation ability of MDS cells. Moreover, in vivo experiments showed METTL14 knockdown remarkably reduced tumor burden and prolonged the survival of mice. Mechanistically, METTL14 facilitated the m6A modification of SETBP1 mRNA by formation of METTL3-METTL14 complex, leading to increased stabilization of SETBP1 mRNA and subsequent activation of the PI3K-AKT signaling pathway. Overall, this study elucidated the involvement of the METTL14/m6A/SETBP1/PI3K-AKT signaling axis in MDS, highlighting the therapeutic potential of targeting METTL3-METTL14 complex-mediated m6A modification for MDS therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


