甲状腺激素调节杏仁核恐惧相关记忆和可塑性的证据
IF 9.6
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
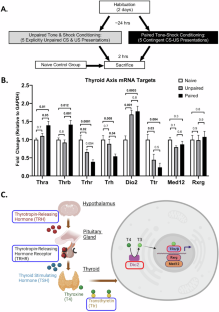
杏仁核是恐惧记忆形成的既定部位,临床研究表明,激素信号级联与创伤相关疾病的发展有关。虽然甲状腺激素(TH)状态与情绪障碍之间的关系已被证实,但相关的脑机制以及甲状腺激素在焦虑症中的作用尚不清楚。在此,我们研究了甲状腺激素受体(TR,未与甲状腺激素结合时为核转录抑制因子,与甲状腺激素结合时为转录激活因子)在杏仁核恐惧记忆最初形成过程中可能起到的介导作用。我们发现在巴甫洛夫恐惧条件反射后,杏仁核中促甲状腺激素释放激素(Trh)、转甲状腺素(Ttr)、促甲状腺激素释放激素受体(Thrr)、2型碘甲腺原氨酸脱碘酶(Dio2)、介导复合体亚基12(Med12/Trap230)和视黄醇X受体γ(Rxrg)等促甲状腺激素释放激素通路调控基因的mRNA水平会发生改变。通过将 TH 激动剂和拮抗剂注入杏仁核,我们证明了这一通路对于恐惧记忆的巩固是必要且充分的。用TR拮抗剂1-850抑制TH信号传导会减少恐惧记忆的巩固;而用T3(三碘甲状腺原氨酸)激活TR则会增加记忆的形成。通过使用全身性甲状腺功能减退的小鼠模型,我们发现杏仁核内输注T3足以挽救恐惧记忆的缺陷。最后,我们证明T3足以激活杏仁核中TR特异性基因通路。这些关于活动依赖性TR调节作用的发现支持了一个模型,即局部TH是杏仁核中恐惧记忆相关可塑性的关键调节因子。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Evidence for thyroid hormone regulation of amygdala-dependent fear-relevant memory and plasticity
The amygdala is an established site for fear memory formation, and clinical studies suggest involvement of hormone signaling cascades in development of trauma-related disorders. While an association of thyroid hormone (TH) status and mood disorders is established, the related brain-based mechanisms and the role of TH in anxiety disorders are unknown. Here we examine the role that TH receptor (TR, a nuclear transcriptional repressor when unbound and a transcriptional activator when bound to TH) may have in mediating the initial formation of fear memories in the amygdala. We identified mRNA levels of TR and other TH pathway regulatory genes, including thyrotropin-releasing hormone (Trh), transthyretin (Ttr), thyrotropin-releasing hormone receptor (Trhr), type 2 iodothyronine deiodinase (Dio2), mediator complex subunit 12 (Med12/Trap230) and retinoid X receptor gamma (Rxrg) to be altered in the amygdala following Pavlovian fear conditioning. Using TH agonist and antagonist infusion into the amygdala, we demonstrated that this pathway is both necessary and sufficient for fear memory consolidation. Inhibition of TH signaling with the TR antagonist 1-850 decreased fear memory consolidation; while activation of TR with T3 (triiodothyronine) resulted in increased memory formation. Using a systemic hypothyroid mouse model, we found that intra-amygdala infusions of T3 were sufficient to rescue deficits in fear memory. Finally, we demonstrated that T3 was sufficient to activate TR-specific gene pathways in the amygdala. These findings on the role of activity-dependent TR modulation support a model in which local TH is a critical regulator of fear memory-related plasticity in the amygdala.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Psychiatry
医学-精神病学
CiteScore
20.50
自引率
4.50%
发文量
459
审稿时长
4-8 weeks
期刊介绍:
Molecular Psychiatry focuses on publishing research that aims to uncover the biological mechanisms behind psychiatric disorders and their treatment. The journal emphasizes studies that bridge pre-clinical and clinical research, covering cellular, molecular, integrative, clinical, imaging, and psychopharmacology levels.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


