在小鼠、大鼠和猕猴体内转导 BBB 穿透型 AAV 向量的能力特征揭示了表达谱的差异。
IF 4.6
3区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
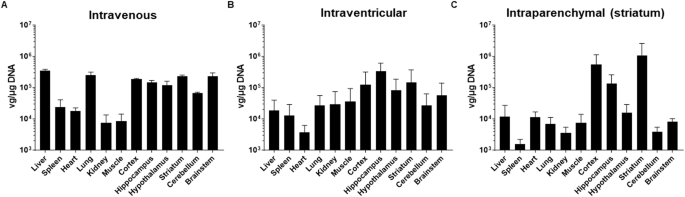
目前正在开发不同的筛选方法,以产生能够在静脉注射时绕过血脑屏障(BBB)的腺相关病毒载体(AAV)。最近,在利用 RNA 驱动的生物扫描技术在 C57BL/6 小鼠体内筛选出的多肽显示载体库中,AAV9P31 脱颖而出,成为最有效的载体。在这项工作中,我们详细描述了它在不同小鼠品系(C57BL/6 和 Balb/c)以及 Sprague Dawley 大鼠和非人灵长类动物(Macaca fascicularis)中的生物分布。通过使用 GFP 和 NanoLuc 报告基因,我们证实了静脉注射 AAV9P31 的小鼠中枢神经系统内感染和转基因表达的均匀性。无论是脑室内注射还是肾实质内注射,都观察到了较为局限的模式。静脉注射后,在小鼠大脑中观察到了不同区域和细胞特异性的转导模式,包括大脑皮层和纹状体中星形胶质细胞和神经元的优先转导,而在海马、丘脑、下丘脑、间脑、脑干和小脑的皮层下位置,神经元是唯一被转导的细胞类型。此外,在中枢神经系统的任何位置都没有发现转导的小胶质细胞。静脉注射转导的外周器官包括肺、肝、腹膜、心脏和骨骼肌。然而,在大鼠和猕猴体内,AAV9P31绕过BBB的性能并不相上下,不过在大鼠脑干静脉注射时发现了较为有限的神经元转导。最后,在猕猴脑室内给药时,皮层、皮层下结构和小脑中的神经元转导呈斑点状。总之,AAV9P31 在小鼠体内静脉注射后可获得广泛的中枢神经系统转导,是模拟各种神经系统疾病的有力工具,也是评估基因治疗疗法的诱人选择。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Characterization of brain transduction capability of a BBB-penetrant AAV vector in mice, rats and macaques reveals differences in expression profiles
Different screening methods are being developed to generate adeno-associated viral vectors (AAV) with the ability to bypass the blood-brain barrier (BBB) upon intravenous administration. Recently, the AAV9P31 stood out as the most efficient version among a library of peptide-displaying capsids selected in C57BL/6 mice using RNA-driven biopanning. In this work we have characterized in detail its biodistribution in different mouse strains (C57BL/6 and Balb/c), as well as in Sprague Dawley rats and non-human primates (Macaca fascicularis). Using GFP and NanoLuc reporter genes, we confirmed homogeneous infection and transgene expression across the CNS of mice injected intravenously with AAV9P31. A more restricted pattern was observed upon either intracerebroventricular or intraparenchymal injection. Following intravenous delivery, region- and cell-specific differential patterns of transduction were observed in the mouse brain, including a preferential transduction of astrocytes and neurons in the cerebral cortex and striatum, whereas neurons were the only transduced cell type in subcortical locations across the hippocampus, thalamus, hypothalamus, mesencephalon, brainstem and cerebellum. Furthermore, transduced microglial cells were never found in any CNS location. Peripheral organs transduced upon intravenous administration included lung, liver, peritoneum, heart and skeletal muscle. However, a comparable performance of AAV9P31 to bypass the BBB in rats and macaques was not observed, although a more limited neuronal transduction was found in the brainstem of rats upon intravenous delivery. Finally, intracerebroventricular delivery in macaques resulted in neuronal transduction in cortical, subcortical structures and cerebellum following a patchy pattern. In conclusion, the widespread CNS transduction obtained in mice upon intravenous delivery of AAV9P31 represents a powerful tool for modeling a wide variety of neurological disorders as well as an appealing choice for the evaluation of gene therapy-based therapeutics.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Gene Therapy
医学-生化与分子生物学
CiteScore
9.70
自引率
2.00%
发文量
67
审稿时长
4-8 weeks
期刊介绍:
Gene Therapy covers both the research and clinical applications of novel therapeutic techniques based on a genetic component. Over the last few decades, significant advances in technologies ranging from identifying novel genetic targets that cause disease through to clinical studies, which show therapeutic benefit, have elevated this multidisciplinary field to the forefront of modern medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


