优化基于肽折叠聚合物的人工逆醛酸酶
IF 4.4
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
肽折叠体具有可预测和可控制的三维结构,是一类有利于开发功能分子的化合物。其中最具挑战性的应用之一是构建类似酶的催化剂。在这里,我们介绍了如何优化由两个 9/12/9/10 螺旋组成的多肽折叠体,使其具有顺式-2-氨基环戊烷羧酸残基的逆醛醇活性。通过对螺旋手性、螺旋间连接刚性和活性位点结构的修改,产生了高活性的逆醛酸酶模拟物。核磁共振测量证实了活性位点残基的假定排列。本文章由计算机程序翻译,如有差异,请以英文原文为准。
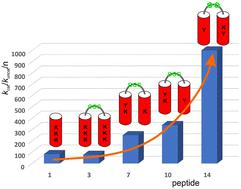
Optimization of peptide foldamer-based artificial retro-aldolase†
Due to their predictable and controllable three-dimensional structure, peptide foldamers constitute a class of compounds beneficial for developing functional molecules. One of the most challenging applications is the construction of enzyme-like catalysts. Here, we describe the optimization of peptide foldamers composed of two 9/12/9/10-helices incorporating cis-2-aminocyclopentanecarboxylic acid residues toward retro-aldol activity. Modifications related to helix handedness, interhelical linker rigidity, and active site construction led to highly active retro-aldolase mimetics. NMR measurements confirmed the assumed arrangement of active site residues.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


