(±)-Tylophorine 的革兰氏级合成
IF 1.7
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们报告了一种实用的可扩展天然产物(±)-tylophorine 的合成方法,该方法采用一种操作简单的无保护基路线,从容易获得的起始原料开始。通过环化合成环状 N-乙酰基二酯化合物,然后经过两个关键步骤(脱羧和克莱门森还原),即可获得目标分子。本文章由计算机程序翻译,如有差异,请以英文原文为准。
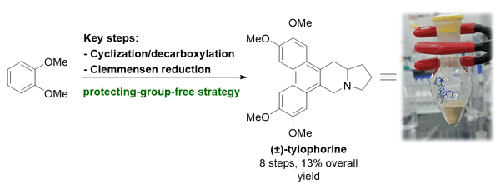
Gram-Scale Synthesis of (±)-Tylophorine
We report a practical scalable synthesis of the natural product (±)-tylophorine by using an operationally simple protecting-group-free route from readily accessible starting materials. Synthesis of a cyclic N-acetyl diester compound through cyclization, followed by two key steps (decarboxylation and a Clemmensen reduction), provides access to the target molecule.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Synlett
化学-有机化学
CiteScore
3.40
自引率
5.00%
发文量
369
审稿时长
1 months
期刊介绍:
SYNLETT is an international journal reporting research results and current trends in chemical synthesis in short personalized reviews and preliminary communications. It covers all fields of scientific endeavor that involve organic synthesis, including catalysis, organometallic, medicinal, biological, and photochemistry, but also related disciplines and offers the possibility to publish scientific primary data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


