肝素和直接凝血酶抑制剂对细胞穿透聚合物 siRNA 转染的影响
IF 4.6
3区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
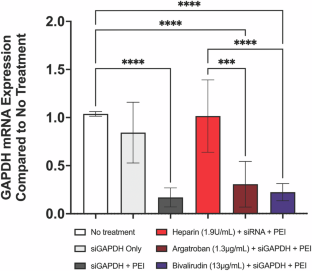
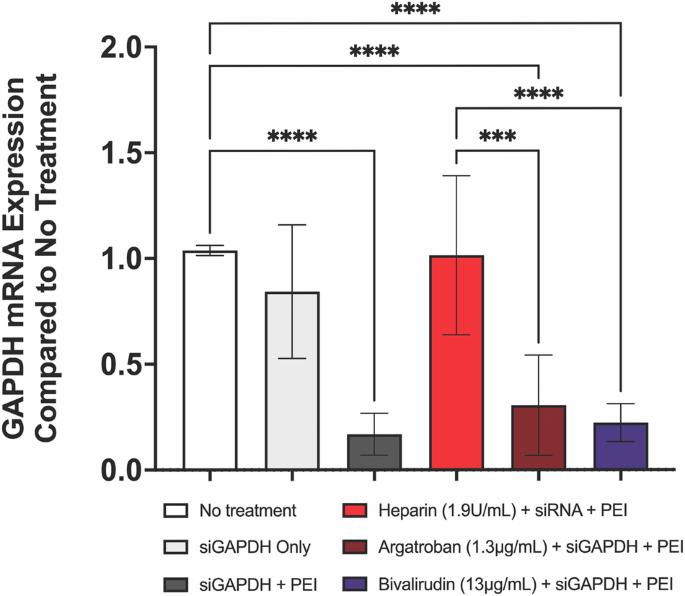
使用 siRNA 进行基因治疗已成为一种很有前景的策略,可实现靶向基因敲除以治疗心血管疾病。然而,高效的 siRNA 转染通常依赖于阳离子递送载体,如合成的细胞穿透聚合物,这些载体容易受到带负电荷分子的干扰。肝素等带负电荷的抗凝剂被广泛应用于心血管领域,可能会严重阻碍 siRNA 的有效递送。因此,我们利用转染了甘油醛-3-磷酸脱氢酶(GAPDH)和β2-微球蛋白(B2M) siRNA 的人平滑肌和内皮细胞,在肝素、阿加曲班和比伐卢定存在下进行了体外研究,以确定哪种抗凝疗法最适合 siRNA 的递送。我们观察到,临床剂量的肝素会降低 siRNA 靶向 mRNA 的敲除效率,而阿加曲班或比伐卢定的存在则不会抑制 mRNA 的敲除。我们的数据表明,使用阳离子转染剂进行 siRNA 治疗时应避免使用肝素,而应使用阿加曲班和比伐卢定。本文章由计算机程序翻译,如有差异,请以英文原文为准。
The impact of heparin and direct thrombin inhibitors on cell-penetrating polymer siRNA transfection
Gene therapy using siRNA has become a promising strategy to achieve targeted gene knockdown for treatment of cardiovascular pathologies. However, efficient siRNA transfection often relies on cationic delivery vectors such as synthetic cell-penetrating polymers which are susceptible to interference by negatively charged molecules. Anticoagulants such as heparin, which is negatively charged and widely used in cardiovascular applications, may pose a significant barrier to effective siRNA delivery. We therefore conducted in vitro studies utilizing human smooth muscle and endothelial cells transfected with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and β2-microglobulin (B2M) siRNA in the presence of heparin, argatroban, and bivalirudin in order to determine which anticoagulant therapy is most compatible for siRNA delivery. We observed that while heparin, at clinical doses, decreases the efficiency of siRNA targeted mRNA knockdown, mRNA knockdown is not inhibited in the presence of either argatroban or bivalirudin. Our data suggests that heparin should be avoided during siRNA therapy with cationic transfection agents, and argatroban and bivalirudin should be used in its stead.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Gene Therapy
医学-生化与分子生物学
CiteScore
9.70
自引率
2.00%
发文量
67
审稿时长
4-8 weeks
期刊介绍:
Gene Therapy covers both the research and clinical applications of novel therapeutic techniques based on a genetic component. Over the last few decades, significant advances in technologies ranging from identifying novel genetic targets that cause disease through to clinical studies, which show therapeutic benefit, have elevated this multidisciplinary field to the forefront of modern medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


