具有类似催化酶的纳米活性和近红外光响应的氧化锌纳米颗粒:有效光动力疗法、自噬、铁变态和抗肿瘤免疫的结合
IF 14.6
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
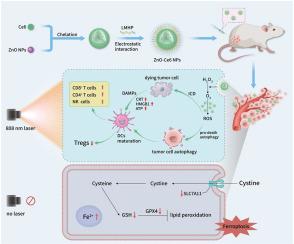
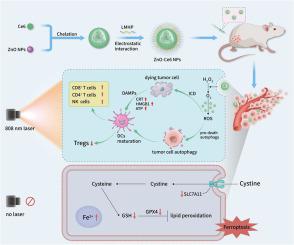
我们制备了具有上转换特性和类似催化酶的纳米酶活性的生物相容性和环境友好型氧化锌纳米颗粒(ZnO NPs)。光动力疗法(PDT)的应用受到紫外可见光穿透性差和肿瘤缺氧环境的严重限制。在这里,我们利用氧化锌纳米粒子作为光敏剂氯素e6(Ce6)的载体,构建了氧化锌-氯素e6纳米粒子(ZnO-Ce6 NPs),同时解决了这两个问题。在穿透性方面,ZnO NPs 可将 808 nm 的近红外光转化为 401 nm 的可见光来激发 Ce6,从而在长波长光下实现深穿透光动力疗法。有趣的是,上转换纳米粒子通常具有在长波长光下发射短波长光的能力。作为纳米酶,氧化锌纳米粒子可以催化肿瘤中过氧化氢的分解,为光动力作用提供氧气,缓解缺氧。增强的光动力作用会产生大量活性氧,过度激活自噬,引发免疫原性细胞死亡(ICD),从而实现抗肿瘤免疫治疗。此外,即使在没有光的情况下,氧化锌和氧化锌-Ce6 NPs 也能诱导肿瘤细胞发生铁变态反应,发挥抗肿瘤作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Zinc oxide nanoparticles with catalase-like nanozyme activity and near-infrared light response: A combination of effective photodynamic therapy, autophagy, ferroptosis, and antitumor immunity
We prepared biocompatible and environment-friendly zinc oxide nanoparticles (ZnO NPs) with upconversion properties and catalase-like nanozyme activity. Photodynamic therapy (PDT) application is severely limited by the poor penetration of UV–Visible light and a hypoxic tumor environment. Here, we used ZnO NPs as a carrier for the photosensitizer chlorin e6 (Ce6) to construct zinc oxide–chlorin e6 nanoparticles (ZnO-Ce6 NPs), simultaneously addressing both problems. In terms of penetration, ZnO NPs convert 808 nm near-infrared light into 401 nm visible light to excite Ce6, achieving deep-penetrating photodynamic therapy under long-wavelength light. Interestingly, the ability to emit short-wavelength light under long-wavelength light is usually observed in upconversion nanoparticles. As nanozymes, ZnO NPs can catalyze the decomposition of hydrogen peroxide in tumors, providing oxygen for photodynamic action and relieving hypoxia. The enhanced photodynamic action produces a large amount of reactive oxygen species, which overactivate autophagy and trigger immunogenic cell death (ICD), leading to antitumor immunotherapy. In addition, even in the absence of light, ZnO and ZnO-Ce6 NPs can induce ferroptosis of tumor cells and exert antitumor effects.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Pharmaceutica Sinica. B
Pharmacology, Toxicology and Pharmaceutics-General Pharmacology, Toxicology and Pharmaceutics
CiteScore
22.40
自引率
5.50%
发文量
1051
审稿时长
19 weeks
期刊介绍:
The Journal of the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association oversees the peer review process for Acta Pharmaceutica Sinica. B (APSB).
Published monthly in English, APSB is dedicated to disseminating significant original research articles, rapid communications, and high-quality reviews that highlight recent advances across various pharmaceutical sciences domains. These encompass pharmacology, pharmaceutics, medicinal chemistry, natural products, pharmacognosy, pharmaceutical analysis, and pharmacokinetics.
A part of the Acta Pharmaceutica Sinica series, established in 1953 and indexed in prominent databases like Chemical Abstracts, Index Medicus, SciFinder Scholar, Biological Abstracts, International Pharmaceutical Abstracts, Cambridge Scientific Abstracts, and Current Bibliography on Science and Technology, APSB is sponsored by the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association. Its production and hosting are facilitated by Elsevier B.V. This collaborative effort ensures APSB's commitment to delivering valuable contributions to the pharmaceutical sciences community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


