建立紫外/可见光谱认知模型
IF 2.9
3区 教育学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
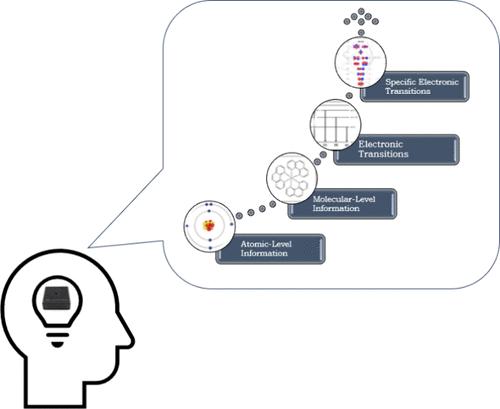
紫外可见光谱法在本科生化学学习中应用广泛。尽管其应用无处不在,但关于学生如何理解或掌握这一光谱工具的研究却很少。为了填补这一空白,我们对中学后化学学生如何理解紫外/可见光谱进行了横断面定性探索性研究,并展示了研究结果。我们采用了现象学建构模型的方法来描述学习者体验紫外/可见光谱现象的不同方式。我们根据复杂程度对这些不同的方式进行了分级。根据学生对光与分子相互作用产生光谱峰的解释,这些层次彼此不同。最低层次的推理显示,学生将光谱峰与分子中的单个原子联系起来。相比之下,最高级别的理解则提供了一种机制,说明光如何通过激活特定电子跃迁的资源,与分子发生特定的相互作用,从而产生光谱峰。这些结果为学生的推理工作模型提供了一个起点。我们将其视为一个发展模型,展示学生如何从原子层面的信息发展到提供光与分子相互作用的机理解释,进而转化为其他基于光-物质相互作用的现象。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Toward a Model of Cognition for UV/Vis Spectroscopy
Ultraviolet–visible spectroscopy is widely used in undergraduate chemistry. Despite its ubiquitous use, very little research has been conducted on how students develop understanding or competency with this spectroscopic tool. To begin addressing this gap, we present results from an exploratory cross-sectional qualitative investigation of how postsecondary chemistry students reason about UV/vis spectroscopy. We used a phenomenographic construct modeling approach to characterize different ways learners experience the phenomenon of UV/vis spectroscopy. We have organized these various ways hierarchically according to sophistication. These levels were distinct from each other based on how students explained light interacting with molecules to give rise to spectral peaks. The lowest level reasoning showed that students link spectral peaks to individual atoms within molecules. In contrast, the highest level of understanding offered a mechanism of how light specifically interacts with molecules to give rise to spectral peaks by activating resources about specific electronic transitions. These results provide a starting point for a working model of students’ reasoning. We view this as a developmental model to show how students can progress from atom-level information to offering a mechanistic explanation of how light interacts with molecules, which can translate to other light–matter interaction-based phenomena.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Chemical Education
化学-化学综合
CiteScore
5.60
自引率
50.00%
发文量
465
审稿时长
6.5 months
期刊介绍:
The Journal of Chemical Education is the official journal of the Division of Chemical Education of the American Chemical Society, co-published with the American Chemical Society Publications Division. Launched in 1924, the Journal of Chemical Education is the world’s premier chemical education journal. The Journal publishes peer-reviewed articles and related information as a resource to those in the field of chemical education and to those institutions that serve them. JCE typically addresses chemical content, activities, laboratory experiments, instructional methods, and pedagogies. The Journal serves as a means of communication among people across the world who are interested in the teaching and learning of chemistry. This includes instructors of chemistry from middle school through graduate school, professional staff who support these teaching activities, as well as some scientists in commerce, industry, and government.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


