消减肠上皮ialylation易导致小鼠急性和慢性肠炎。
IF 7.1
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
Cellular and Molecular Gastroenterology and Hepatology
Pub Date : 2024-01-01
DOI:10.1016/j.jcmgh.2024.101378
引用次数: 0
摘要
背景和目的:在糖共轭物中添加硅烷基酸(硅烷基化)是糖基化的一个常见封顶步骤。我们的研究旨在确定整个糖基化在肠粘膜稳态中的作用:方法:通过体外研究和突变组学研究,产生了构成性缺失肠上皮糖基化的小鼠(IEC Slc35a1-/-小鼠)和诱导性缺失肠上皮糖基化的小鼠(TM-IEC Slc35a1-/-小鼠),用于确定整体糖基化在肠粘膜稳态中的作用:结果:IEC Slc35a1-/- 小鼠出现了轻度自发性微生物群依赖性结肠炎。此外,30%的 IEC Slc35a1-/- 小鼠在 12 个月大时直肠内出现自发性肿瘤。与对照组相比,TM-IEC Slc35a1-/-小鼠极易受1% DSS诱导的急性炎症影响。总ialylation的丧失与粪便切片和结肠组织内粘液厚度的降低有关。TM-IEC Slc35a1-/- 小鼠的微生物群发生了改变,梭状芽孢杆菌(Clostridia disporicum)增加,这与至少 20 个独特类群丰度的全面降低有关;但是,代谢组学分析并未显示短链脂肪酸水平有任何显著差异。用5-氟尿嘧啶(5-FU)治疗会导致IEC Slc35a1-/-小鼠的小肠粘膜炎比WT同窝小鼠更严重,这与IEC Slc35a1-/-;Lgr5-GFP小鼠小肠隐窝中Lgr5+细胞数量减少有关:结论:整体硅烷基化的缺失会损害粘液稳定性和干细胞生态位,导致微生物群依赖性自发性结肠炎和肿瘤发生。本文章由计算机程序翻译,如有差异,请以英文原文为准。
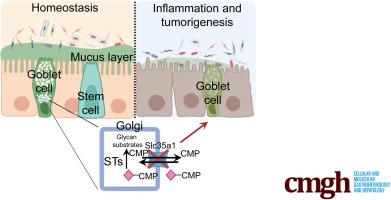
Ablation of Intestinal Epithelial Sialylation Predisposes to Acute and Chronic Intestinal Inflammation in Mice
Background & Aims
Addition of sialic acids (sialylation) to glycoconjugates is a common capping step of glycosylation. Our study aims to determine the roles of the overall sialylation in intestinal mucosal homeostasis.
Methods
Mice with constitutive deletion of intestinal epithelial sialylation (IEC Slc35a1–/– mice) and mice with inducible deletion of sialylation in intestinal epithelium (TM-IEC Slc35a1–/– mice) were generated, which were used to determine the roles of overall sialylation in intestinal mucosal homeostasis by ex vivo and mutiomics studies.
Results
IEC Slc35a1–/– mice developed mild spontaneous microbiota-dependent colitis. Additionally, 30% of IEC Slc35a1–/– mice had spontaneous tumors in the rectum greater than the age of 12 months. TM-IEC Slc35a1–/– mice were highly susceptible to acute inflammation induced by 1% dextran sulfate sodium versus control animals. Loss of total sialylation was associated with reduced mucus thickness on fecal sections and within colon tissues. TM-IEC Slc35a1–/– mice showed altered microbiota with an increase in Clostridium disporicum, which is associated a global reduction in the abundance of at least 10 unique taxa; however, metabolomic analysis did not show any significant differences in short-chain fatty acid levels. Treatment with 5-fluorouracil led to more severe small intestine mucositis in the IEC Slc35a1–/– mice versus wild-type littermates, which was associated with reduced Lgr5+ cell representation in small intestinal crypts in IEC Slc35a1-/-;Lgr5-GFP mice.
Conclusions
Loss of overall sialylation impairs mucus stability and the stem cell niche leading to microbiota-dependent spontaneous colitis and tumorigenesis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cellular and Molecular Gastroenterology and Hepatology
Medicine-Gastroenterology
CiteScore
13.00
自引率
2.80%
发文量
246
审稿时长
42 days
期刊介绍:
"Cell and Molecular Gastroenterology and Hepatology (CMGH)" is a journal dedicated to advancing the understanding of digestive biology through impactful research that spans the spectrum of normal gastrointestinal, hepatic, and pancreatic functions, as well as their pathologies. The journal's mission is to publish high-quality, hypothesis-driven studies that offer mechanistic novelty and are methodologically robust, covering a wide range of themes in gastroenterology, hepatology, and pancreatology.
CMGH reports on the latest scientific advances in cell biology, immunology, physiology, microbiology, genetics, and neurobiology related to gastrointestinal, hepatobiliary, and pancreatic health and disease. The research published in CMGH is designed to address significant questions in the field, utilizing a variety of experimental approaches, including in vitro models, patient-derived tissues or cells, and animal models. This multifaceted approach enables the journal to contribute to both fundamental discoveries and their translation into clinical applications, ultimately aiming to improve patient care and treatment outcomes in digestive health.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


