一项开放标签、随机对照试验,对脓毒症重症患者的生酮饮食进行评估。
IF 15.8
1区 医学
Q1 CELL BIOLOGY
引用次数: 0
摘要
脓毒症患者会出现新陈代谢和免疫功能障碍,而标准的碳水化合物营养可能会加重这种症状。尽管临床证据有限,但生酮饮食(KD)可能提供一种免疫优势的替代方案。我们进行了一项单中心、开放标签、随机对照试验,以评估生酮饮食能否诱导脓毒症重症患者出现稳定的酮病。次要结果包括评估 KD 的可行性和安全性,以及对临床和免疫学特征的探索性分析。40 名成人重症患者被随机分配到生酮饮食或标准高碳水化合物饮食中。所有 KD 患者都达到了稳定的酮病状态,与对照组相比,β-羟丁酸水平显著增加[平均差异为每升 1.4 毫摩尔;95% 置信区间 (CI):1.0 至 1.8;P < 0.001]。没有观察到重大不良事件或有害的代谢副作用(酸中毒、血糖异常或血脂异常)。第 4 天后,KD 组患者无一需要胰岛素治疗,而对照组患者的胰岛素依赖度在 35% 到 60% 之间(P = 0.009)。30 天存活率没有差异,但无通气[发生率比 (IRR) 1.7; 95% CI: 1.5 to 2.1; P < 0.001]、无血管加压(IRR 1.7; 95% CI: 1.5 to 2.0; P < 0.001)、无透析(IRR 1.5; 95% CI: 1.3 to 1.8; P < 0.001)和无重症监护室天数(IRR 1.7; 95% CI: 1.4 to 2.1; P < 0.001)均高于生酮组。CD4+/CD8+ T细胞的下一代测序和蛋白质分析表明,免疫失调减少,T细胞活化和信号标记基因表达降低,促炎细胞因子分泌减少。这项试验证明了在脓毒症患者中诱导稳定生酮状态是安全的,因此有必要进行更大规模的试验,研究其对脓毒症相关器官功能障碍的潜在益处。本文章由计算机程序翻译,如有差异,请以英文原文为准。
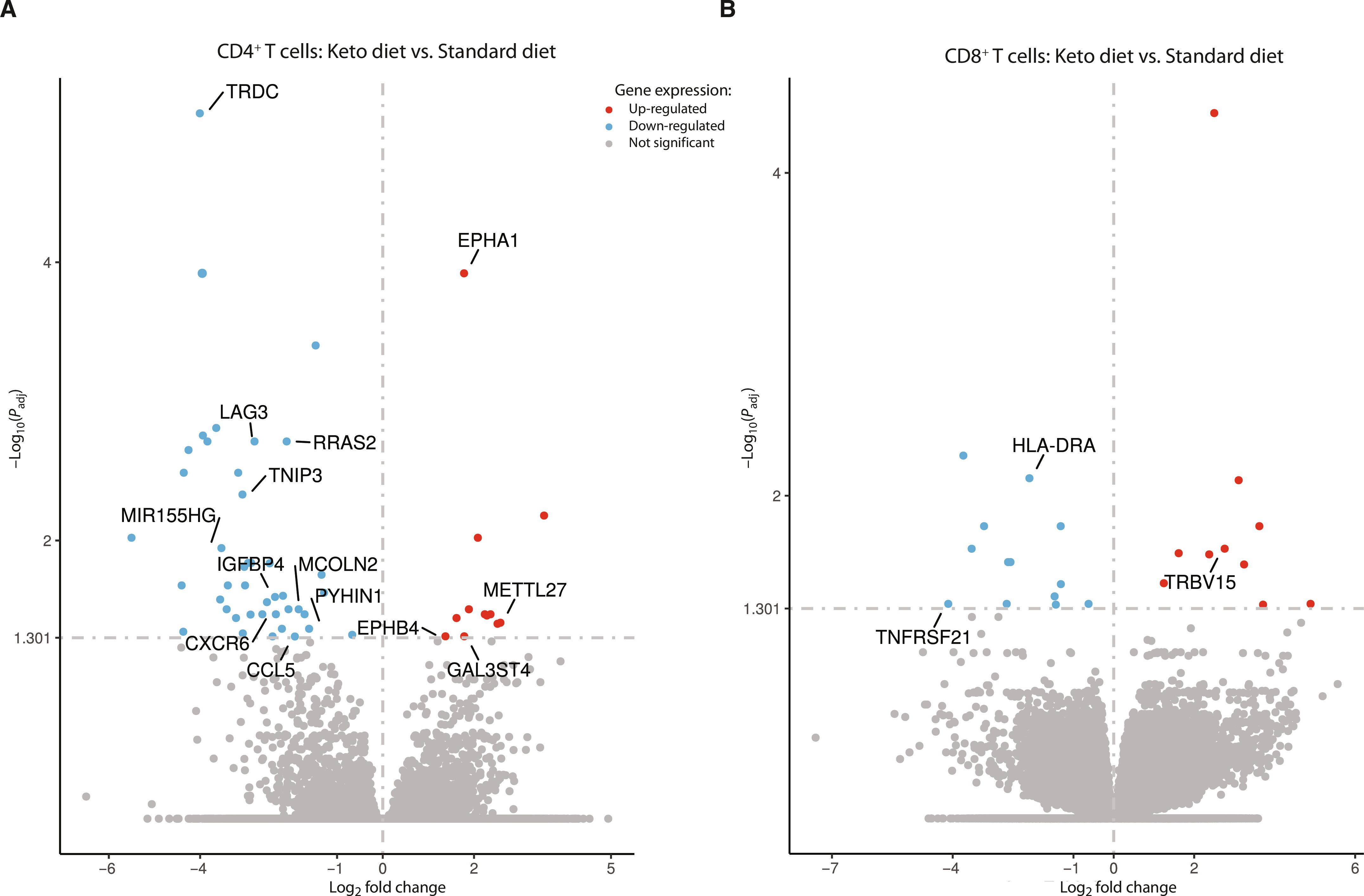
An open-label, randomized controlled trial to assess a ketogenic diet in critically ill patients with sepsis
Patients with sepsis experience metabolic and immunologic dysfunction that may be amplified by standard carbohydrate-based nutrition. A ketogenic diet (KD) may offer an immunologically advantageous alternative, although clinical evidence is limited. We conducted a single-center, open-label, randomized controlled trial to assess whether a KD could induce stable ketosis in critically ill patients with sepsis. Secondary outcomes included assessment of feasibility and safety of KD, as well as explorative analysis of clinical and immunological characteristics. Forty critically ill adults were randomized to either a ketogenic or standard high-carbohydrate diet. Stable ketosis was achieved in all KD patients, with significant increases in β-hydroxybutyrate levels compared with controls [mean difference 1.4 milimoles per liter; 95% confidence interval (CI): 1.0 to 1.8; P < 0.001). No major adverse events or harmful metabolic side effects (acidosis, dysglycemia, or dyslipidemia) were observed. After day 4, none of the patients in the KD group required insulin treatment, whereas in the control group, insulin dependency ranged between 35% and 60% (P = 0.009). There were no differences in 30-day survival, but ventilation-free [incidence rate ratio (IRR) 1.7; 95% CI: 1.5 to 2.1; P < 0.001], vasopressor-free (IRR 1.7; 95% CI: 1.5 to 2.0; P < 0.001), dialysis-free (IRR 1.5; 95% CI: 1.3 to 1.8; P < 0.001), and intensive care unit–free days (IRR 1.7; 95% CI: 1.4 to 2.1; P < 0.001) were higher in the ketogenic group. Next-generation sequencing of CD4+/CD8+ T cells and protein analyses showed reduced immune dysregulation, with decreased gene expression of T-cell activation and signaling markers and lower pro-inflammatory cytokine secretion. This trial demonstrated the safe induction of a stable ketogenic state in sepsis, warranting larger trials to investigate potential benefits in sepsis-related organ dysfunction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science Translational Medicine
CELL BIOLOGY-MEDICINE, RESEARCH & EXPERIMENTAL
CiteScore
26.70
自引率
1.20%
发文量
309
审稿时长
1.7 months
期刊介绍:
Science Translational Medicine is an online journal that focuses on publishing research at the intersection of science, engineering, and medicine. The goal of the journal is to promote human health by providing a platform for researchers from various disciplines to communicate their latest advancements in biomedical, translational, and clinical research.
The journal aims to address the slow translation of scientific knowledge into effective treatments and health measures. It publishes articles that fill the knowledge gaps between preclinical research and medical applications, with a focus on accelerating the translation of knowledge into new ways of preventing, diagnosing, and treating human diseases.
The scope of Science Translational Medicine includes various areas such as cardiovascular disease, immunology/vaccines, metabolism/diabetes/obesity, neuroscience/neurology/psychiatry, cancer, infectious diseases, policy, behavior, bioengineering, chemical genomics/drug discovery, imaging, applied physical sciences, medical nanotechnology, drug delivery, biomarkers, gene therapy/regenerative medicine, toxicology and pharmacokinetics, data mining, cell culture, animal and human studies, medical informatics, and other interdisciplinary approaches to medicine.
The target audience of the journal includes researchers and management in academia, government, and the biotechnology and pharmaceutical industries. It is also relevant to physician scientists, regulators, policy makers, investors, business developers, and funding agencies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


