通过氮丙啶化/氮丙啶打开协议对部分功能化环烯支架进行氟化转化的尝试
IF 1.7
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本研究介绍了通过环烯烃键氮丙啶化/氮丙啶开环与氟化作用对一些官能化环烯烃衍生物进行转化的研究。被选中进行氟化官能化的模型化合物是具有环己烯骨架的氨基酯和二酯以及环戊烯融合的β-内酰胺。官能化是通过底物导向的非对映选择性烯烃键氮丙啶化作用进行的,然后是氟化物介导的氮丙啶开环或分子内内酯化作用,得到一些氟化的氨基酯或氨基内酯衍生物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
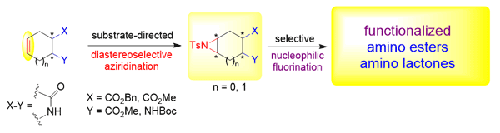
Attempts on Fluorinative Transformation of Selected Functionalized Cycloalkene Scaffolds through Aziridination/Aziridine-Opening Protocol
Studies on the transformations of some functionalized cycloalkene derivatives through their ring olefin-bond aziridination/aziridine opening with fluoride are presented. The selected model compounds submitted to fluorinative functionalization were an amino ester and diesters with a cyclohexene skeleton as well as a cyclopentene-fused β-lactam. Functionalization proceeded across a substrate-directed diastereoselective olefin-bond aziridination, followed by fluoride-mediated aziridine opening or intramolecular lactonization giving some fluorinated amino ester or amino lactone derivatives.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Synlett
化学-有机化学
CiteScore
3.40
自引率
5.00%
发文量
369
审稿时长
1 months
期刊介绍:
SYNLETT is an international journal reporting research results and current trends in chemical synthesis in short personalized reviews and preliminary communications. It covers all fields of scientific endeavor that involve organic synthesis, including catalysis, organometallic, medicinal, biological, and photochemistry, but also related disciplines and offers the possibility to publish scientific primary data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


