(-)-Deglycocadambine 的全合成
IF 4.6
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
以 (+)-genipin 为手性起始原料,通过 12 个步骤首次实现了单萜吲哚生物碱 (-)-deglycocadambine 的全合成。所报道的合成方法的特点是在色胺和高度官能化的二醛前体之间通过精心策划的级联环化反应,以聚合的方式快速引入独特的 6/5/6/7/6 融合五环骨架和位于 C19 的官能团酮。在后期合成过程中,成功实现了 C3 处的跨annular 氧化环化以形成桥接的恶唑烷,从而确保了该分子的最终全合成。本文章由计算机程序翻译,如有差异,请以英文原文为准。
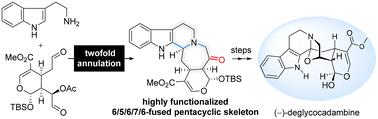
Total synthesis of (–)-deglycocadambine†
The first total synthesis of the monoterpene indole alkaloid (–)-deglycocadambine is achieved in 12 steps with (+)-genipin as the chiral starting material. The reported synthetic approach is characterized by an orchestrated cascade annulation between tryptamine and the highly functionalized dialdehyde precursor, rapidly introducing the unique 6/5/6/7/6-fused pentacyclic skeleton and the ketone functional group at C19 in a convergent manner. The successful implementation of transannular oxidative cyclization at C3 for bridged oxazolidine formation in the late-stage synthetic campaign ensured the final total synthesis of this molecule.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Chemistry Frontiers
CHEMISTRY, ORGANIC-
CiteScore
7.90
自引率
11.10%
发文量
686
审稿时长
1 months
期刊介绍:
Organic Chemistry Frontiers is an esteemed journal that publishes high-quality research across the field of organic chemistry. It places a significant emphasis on studies that contribute substantially to the field by introducing new or significantly improved protocols and methodologies. The journal covers a wide array of topics which include, but are not limited to, organic synthesis, the development of synthetic methodologies, catalysis, natural products, functional organic materials, supramolecular and macromolecular chemistry, as well as physical and computational organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


