地球富金属催化的 1-溴环丁烯与格氏试剂的交叉偶联反应
IF 1.7
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
利用两种有效催化剂(如 Fe(acac)3 和 Ni(acac)2),在 C2 取代的 1-bromocyclobut-1-enes 和格氏试剂之间建立了交叉偶联反应。铁催化剂可在 THF 中使用,但需要 NMP 作为助溶剂,其优点是可以与烷基格氏试剂发生交叉偶联反应。镍催化剂无需任何添加剂即可在 THF 中促进反应,并与富电子芳基格氏试剂发生高反应性。这些催化剂以良好的收率生成了各种类型的取代环丁烯。本文章由计算机程序翻译,如有差异,请以英文原文为准。
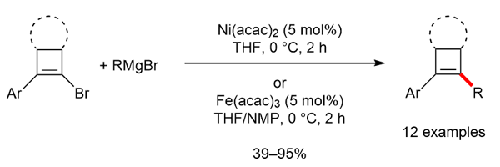
Earth-Abundant Metal-Catalyzed Cross-Coupling Reactions of 1-Bromocyclobut-1-enes with Grignard Reagents
Cross-coupling reactions have been developed between C2-substituted 1-bromocyclobut-1-enes and Grignard reagents using two effective catalysts, e.g., Fe(acac)3 and Ni(acac)2. The iron catalyst works in THF but requires NMP as the co-solvent, with the advantage of achieving cross-coupling reactions with alkyl Grignard reagents. The nickel catalyst was able to promote the reactions in THF without any additive and showed high reactivity with electron-rich aryl Grignard reagents. These catalysts gave various types of substituted cyclobutenes in good yields.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Synlett
化学-有机化学
CiteScore
3.40
自引率
5.00%
发文量
369
审稿时长
1 months
期刊介绍:
SYNLETT is an international journal reporting research results and current trends in chemical synthesis in short personalized reviews and preliminary communications. It covers all fields of scientific endeavor that involve organic synthesis, including catalysis, organometallic, medicinal, biological, and photochemistry, but also related disciplines and offers the possibility to publish scientific primary data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


