电荷引导的膜失稳肽掩蔽技术可实现高效的内泌体逃逸,从而在细胞内定向输送蛋白质
IF 14.7
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
肽、蛋白质和核酸等生物物质的细胞内输送为创新疗法带来了巨大机遇。然而,内质体诱捕仍然是生物大分子细胞内递送的主要瓶颈,在很大程度上限制了它们的治疗潜力。在这里,我们通过用具有 pH 响应性的反离子肽掩蔽低分子量原胺(LMWP),将细胞穿透肽(CPP)转化为内质体逃逸肽(EEPs)。由此产生的屏蔽 CPPs(mLMWP 和 mLMWP2)有效地促进了肽/蛋白质货物从内体逃逸到细胞质中。内溶酶体泄漏后,溶酶体修复和溶酶吞噬作用随之启动。活性氧(ROS)升高或细胞死亡的情况极少。在 mLMWP2 的基础上,我们构建了一种细胞内蛋白质递送系统,其中包含作为靶向模块的抗体、作为内溶酶体逸出模块的 mLMWP2 以及所需的蛋白质货物。通过这种 HER2 靶向递送系统,我们将环化重组酶(Cre)和 BH3-相互作用结构域死亡激动剂(BID)有效地转运到 HER2 细胞的细胞质中,从而发挥其生物活性。因此,模块化递送系统显示出其作为科学研究和治疗应用工具的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
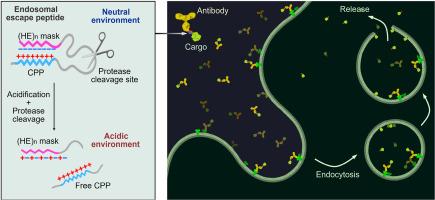
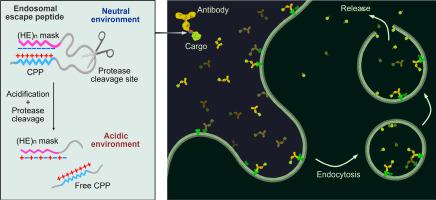
Charge-guided masking of a membrane-destabilizing peptide enables efficient endosomal escape for targeted intracellular delivery of proteins
Intracellular delivery of biologicals such as peptides, proteins, and nucleic acids presents a great opportunity for innovative therapeutics. However, the endosome entrapment remains a major bottleneck in the intracellular delivery of biomacromolecules, largely limiting their therapeutic potential. Here, we converted a cell-penetrating peptide (CPP), low molecular weight protamine (LMWP), to endosomal escape peptides (EEPs) by masking LMWP with a pH-responsive counter-ionic peptide. The resulting masked CPPs (mLMWP and mLMWP2) effectively promoted the escape of peptide/protein cargoes from endosomes into the cytoplasm. Consequential lysosome repair and lysophagy were initiated upon the endolysosomal leakage. Minimal reactive oxygen species (ROS) elevation or cell death was observed. Based on mLMWP2, we constructed an intracellular protein delivery system containing an antibody as a targeting module, mLMWP2 as an endosomal escape module, and the desired protein cargo. With the HER2-targeting delivery system, we efficiently translocated cyclization recombination enzyme (Cre) and BH3-interacting domain death agonist (BID) into the cytosol of HER2+ cells to exert their biological activity. Thereby, the modular delivery system shows its potential as a promising tool for scientific studies and therapeutic applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Pharmaceutica Sinica. B
Pharmacology, Toxicology and Pharmaceutics-General Pharmacology, Toxicology and Pharmaceutics
CiteScore
22.40
自引率
5.50%
发文量
1051
审稿时长
19 weeks
期刊介绍:
The Journal of the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association oversees the peer review process for Acta Pharmaceutica Sinica. B (APSB).
Published monthly in English, APSB is dedicated to disseminating significant original research articles, rapid communications, and high-quality reviews that highlight recent advances across various pharmaceutical sciences domains. These encompass pharmacology, pharmaceutics, medicinal chemistry, natural products, pharmacognosy, pharmaceutical analysis, and pharmacokinetics.
A part of the Acta Pharmaceutica Sinica series, established in 1953 and indexed in prominent databases like Chemical Abstracts, Index Medicus, SciFinder Scholar, Biological Abstracts, International Pharmaceutical Abstracts, Cambridge Scientific Abstracts, and Current Bibliography on Science and Technology, APSB is sponsored by the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association. Its production and hosting are facilitated by Elsevier B.V. This collaborative effort ensures APSB's commitment to delivering valuable contributions to the pharmaceutical sciences community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


