疾病衍生循环细胞外囊泡预处理:间充质干细胞精准治疗的可行策略
IF 14.7
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
基于间充质干细胞(MSC)的疗法已成为前景广阔的再生医学方法;然而,如何精确增强其组织修复效果仍是该领域的一个重大问题。来自疾病状态的循环细胞外囊泡(EVs)携带多种病理信息,并影响受体细胞的功能。基于这种独特的特性,我们报告说,疾病衍生循环EV(disease-EV)预处理是一种有效的策略,可精确增强间充质干细胞在不同疾病模型中的组织修复能力。简而言之,来自肺或肾组织损伤的血浆 EV 含有独特的富集分子,并能在培养的间充质干细胞中诱导组织损伤特异性基因表达反应。疾病-EV预处理通过代谢重编程(如增强氧化磷酸化和脂质代谢)改善了间充质干细胞的性能(包括增殖、迁移和生长因子的产生),而不会诱导不良的免疫反应。因此,与正常间充质干细胞相比,经疾病-EV 预处理的间充质干细胞在不同类型的组织损伤(如急性肺损伤或肾损伤)中表现出卓越的组织修复效果(包括抗炎和抗凋亡作用)。疾病衍生的EV具有多种优势,如灵活性、稳定性、长期储存以及易于运输和使用,因此可作为一种 "现成 "产品。这项研究强调了疾病-EV预处理是精确增强间充质干细胞疗法再生能力的有力策略这一观点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
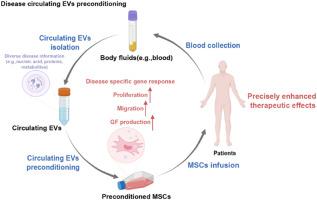
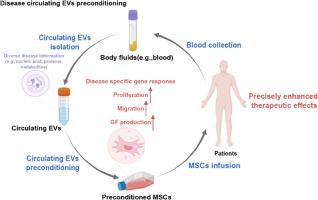
Disease-derived circulating extracellular vesicle preconditioning: A promising strategy for precision mesenchymal stem cell therapy
Mesenchymal stem cell (MSC)-based therapies have emerged as promising methods for regenerative medicine; however, how to precisely enhance their tissue repair effects is still a major question in the field. Circulating extracellular vesicles (EVs) from diseased states carry diverse pathological information and affect the functions of recipient cells. Based on this unique property, we report that disease-derived circulating EV (disease-EV) preconditioning is a potent strategy for precisely enhancing the tissue repair potency of MSCs in diverse disease models. Briefly, plasma EVs from lung or kidney tissue injuries were shown to contain distinctly enriched molecules and were shown to induce tissue injury-specific gene expression responses in cultured MSCs. Disease-EV preconditioning improved the performance (including proliferation, migration, and growth factor production) of MSCs through metabolic reprogramming (such as via enhanced oxidative phosphorylation and lipid metabolism) without inducing an adverse immune response. Consequently, compared with normal MSCs, disease-EV-preconditioned MSCs exhibited superior tissue repair effects (including anti-inflammatory and antiapoptotic effects) in diverse types of tissue injury (such as acute lung or kidney injury). Disease-derived EVs may serve as a type of “off-the-shelf” product due to multiple advantages, such as flexibility, stability, long-term storage, and ease of shipment and use. This study highlights the idea that disease-EV preconditioning is a robust strategy for precisely enhancing the regenerative capacity of MSC-based therapies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Pharmaceutica Sinica. B
Pharmacology, Toxicology and Pharmaceutics-General Pharmacology, Toxicology and Pharmaceutics
CiteScore
22.40
自引率
5.50%
发文量
1051
审稿时长
19 weeks
期刊介绍:
The Journal of the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association oversees the peer review process for Acta Pharmaceutica Sinica. B (APSB).
Published monthly in English, APSB is dedicated to disseminating significant original research articles, rapid communications, and high-quality reviews that highlight recent advances across various pharmaceutical sciences domains. These encompass pharmacology, pharmaceutics, medicinal chemistry, natural products, pharmacognosy, pharmaceutical analysis, and pharmacokinetics.
A part of the Acta Pharmaceutica Sinica series, established in 1953 and indexed in prominent databases like Chemical Abstracts, Index Medicus, SciFinder Scholar, Biological Abstracts, International Pharmaceutical Abstracts, Cambridge Scientific Abstracts, and Current Bibliography on Science and Technology, APSB is sponsored by the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association. Its production and hosting are facilitated by Elsevier B.V. This collaborative effort ensures APSB's commitment to delivering valuable contributions to the pharmaceutical sciences community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


