电化学膨胀石墨烯片亚纳米级夹层中的空间限制自由基加成反应
IF 6.7
2区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
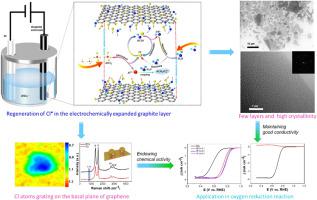
在这里,我们报告了在石墨的电化学剥离过程中,膨胀石墨烯片的亚纳米级夹层中发生的空间限制自由基加成反应。由于石墨烯的化学稳定性,在制备过程中同时对石墨烯进行功能化仍是一项挑战。为此,我们使用四氯铝酸盐(AlCl)和硫酸盐(SO)作为石墨电化学剥离的共闰阴离子。我们发现 AlCl 离子在石墨层中的插层极不可逆,并在电解过程中生成了 C-Cl 键。氯和氧均匀地分布在所获得石墨烯的基底面上,并且通过调节电解质溶液中 AlCl 阴离子的浓度实现了可控的功能化,这表明在膨胀石墨的亚纳米层间发生了空间限制的氯加成反应。此外,氯氧杂化取代石墨烯在氧还原反应中表现出优异的电催化性能。这项工作展示了在纳米或亚纳米尺度的密闭空间内发生的创新性自由基加成反应,同时还提供了在石墨电化学剥离过程中直接对石墨烯进行功能化的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Spatially confined radical addition reaction in the sub-nanometer scaled interlayers of electrochemically expanded graphene sheets
Here we report a spatially confined radical addition reaction which occurs in the sub-nanometer scaled interlayers of the expanded graphene sheets during the electrochemical exfoliation processes of graphite. Due to its chemical stability, challenge remains to functionalize graphene simultaneously during the preparation processes. To this, we use tetrachloroaluminate (AlCl) as the co-intercalation anions together with sulfate (SO) for the electrochemical exfoliation of graphite. We revealed the extremely irreversible intercalation of AlCl ions in graphite layers and the generation of C–Cl bonds during electrolysis. Chlorine and oxygen were homogeneously distributed on the basal plane of the obtained graphene, and the controlled functionalization was achieved by tuning the concentration of AlCl anions in the electrolyte solution, indicating the spatially confined chlorine addition reaction occurring between the sub-nanometer interlayers of expanded graphite. Furthermore, the chlorine and oxygen hybrid-substituted graphene exhibited excellent electrocatalytic performance for oxygen reduction reaction. This work demonstrates an innovative radical addition reaction in the confined space at nanometer or subnanometer scale and, meanwhile, provides a direct functionalization of graphene during the electrochemical exfoliation of graphite.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today Chemistry
Multiple-
CiteScore
8.90
自引率
6.80%
发文量
596
审稿时长
33 days
期刊介绍:
Materials Today Chemistry is a multi-disciplinary journal dedicated to all facets of materials chemistry.
This field represents one of the fastest-growing areas of science, involving the application of chemistry-based techniques to the study of materials. It encompasses materials synthesis and behavior, as well as the intricate relationships between material structure and properties at the atomic and molecular scale. Materials Today Chemistry serves as a high-impact platform for discussing research that propels the field forward through groundbreaking discoveries and innovative techniques.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


