酸性鞘磷脂酶与溶酶体膜和阳离子两亲药物的相互作用:分子动力学研究
IF 4.4
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Computational and structural biotechnology journal
Pub Date : 2024-06-02
DOI:10.1016/j.csbj.2024.05.049
引用次数: 0
摘要
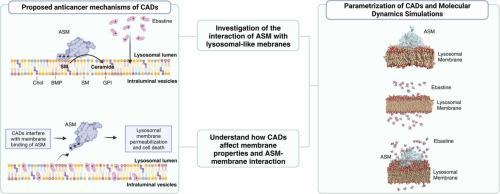
溶酶体在细胞功能和疾病中起着举足轻重的作用,通过酸性鞘磷脂酶(ASM)调节溶酶体膜的完整性,影响癌症的进展和耐药性。此外,阳离子两亲药物(CADs)是已知的ASM抑制剂,具有抗癌活性,但它们与溶酶体膜和ASM相互作用的结构机制却鲜为人知。我们的研究利用全原子显式溶剂分子动力学模拟,深入研究了糖基化的 ASM 与溶酶体膜的相互作用,以及 CAD 代表药物(即依巴斯汀、羟基巴斯汀和氯雷他定)对溶酶体膜和 ASM 的影响。我们的研究结果证实了 ASM 是通过沙波素结构域与膜结合的,这在以前的粗粒度模型中仅有所显示。此外,我们还阐明了特定残基和 ASM 诱导的膜曲率在脂质招募和定向中的作用。CADs 还在催化结构域中参与膜相互作用的环的水平上干扰了 ASM 与膜的结合。我们的计算方法适用于不同的 CAD 或膜组成,为了解 ASM 和 CAD 与膜的相互作用提供了见解,为今后的研究提供了宝贵的工具。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Acidic sphingomyelinase interactions with lysosomal membranes and cation amphiphilic drugs: A molecular dynamics investigation
Lysosomes are pivotal in cellular functions and disease, influencing cancer progression and therapy resistance with Acid Sphingomyelinase (ASM) governing their membrane integrity. Moreover, cation amphiphilic drugs (CADs) are known as ASM inhibitors and have anti-cancer activity, but the structural mechanisms of their interactions with the lysosomal membrane and ASM are poorly explored. Our study, leveraging all-atom explicit solvent molecular dynamics simulations, delves into the interaction of glycosylated ASM with the lysosomal membrane and the effects of CAD representatives, i.e., ebastine, hydroxyebastine and loratadine, on the membrane and ASM. Our results confirm the ASM association to the membrane through the saposin domain, previously only shown with coarse-grained models. Furthermore, we elucidated the role of specific residues and ASM-induced membrane curvature in lipid recruitment and orientation. CADs also interfere with the association of ASM with the membrane at the level of a loop in the catalytic domain engaging in membrane interactions. Our computational approach, applicable to various CADs or membrane compositions, provides insights into ASM and CAD interaction with the membrane, offering a valuable tool for future studies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Computational and structural biotechnology journal
Biochemistry, Genetics and Molecular Biology-Biophysics
CiteScore
9.30
自引率
3.30%
发文量
540
审稿时长
6 weeks
期刊介绍:
Computational and Structural Biotechnology Journal (CSBJ) is an online gold open access journal publishing research articles and reviews after full peer review. All articles are published, without barriers to access, immediately upon acceptance. The journal places a strong emphasis on functional and mechanistic understanding of how molecular components in a biological process work together through the application of computational methods. Structural data may provide such insights, but they are not a pre-requisite for publication in the journal. Specific areas of interest include, but are not limited to:
Structure and function of proteins, nucleic acids and other macromolecules
Structure and function of multi-component complexes
Protein folding, processing and degradation
Enzymology
Computational and structural studies of plant systems
Microbial Informatics
Genomics
Proteomics
Metabolomics
Algorithms and Hypothesis in Bioinformatics
Mathematical and Theoretical Biology
Computational Chemistry and Drug Discovery
Microscopy and Molecular Imaging
Nanotechnology
Systems and Synthetic Biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


