端基炔烃的串联二重化-硼化反应:制备 α-取代烯基硼酸盐的实用途径
IF 1.7
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本文介绍了一种将末端炔烃催化转化为 α-取代乙烯基硼酸酯的实用方法。该工艺采用催化量的纳米颗粒支撑金催化剂和催化量的铜来实现整体转化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
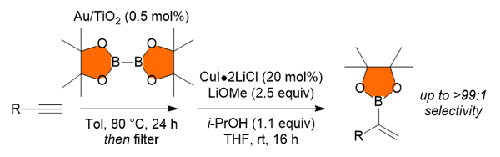
Tandem Diboration–Protoboration of Terminal Alkynes: A Practical Route to α-Substituted Alkenyl Boronates
A practical method is introduced for the catalytic conversion of terminal alkynes into α-substituted vinyl boronic esters. The process employs catalytic amounts of nanoparticle-supported gold catalysts and catalytic amounts of copper to effect the overall transformation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Synlett
化学-有机化学
CiteScore
3.40
自引率
5.00%
发文量
369
审稿时长
1 months
期刊介绍:
SYNLETT is an international journal reporting research results and current trends in chemical synthesis in short personalized reviews and preliminary communications. It covers all fields of scientific endeavor that involve organic synthesis, including catalysis, organometallic, medicinal, biological, and photochemistry, but also related disciplines and offers the possibility to publish scientific primary data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


