3-螺蒽取代的 1,3,4-噻二唑啉类化合物
IF 1
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
通过类固醇 3-酮与氨基甲酸硫酰肼的反应,开发了一种通过环 A 合成 1,3,4-噻二唑啉螺类固醇的方法。结果表明,如果前体类固醇的环 A 和环 D 中都有酮基,则反应只发生在环 A 的酮基上。本文章由计算机程序翻译,如有差异,请以英文原文为准。
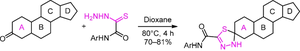
3-Spiroandrostene-substituted 1,3,4-thiadiazolines
A method for the synthesis of 1,3,4-thiadiazoline spiro steroids via ring A by the reaction of steroid 3-ketones with oxamic acid thiohydrazides was developed. It was shown that if a keto group was present both in ring A and in ring D of the precursor steroid, the reaction occurred only at the keto group of ring A. A number of new steroidal 3-spiroandrostene-substituted 1,3,4-thiadiazolines were obtained, which could be easily acetylated at the thiadiazole NH group.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
2.90
自引率
13.30%
发文量
98
审稿时长
1 months
期刊介绍:
The international journal Chemistry of Heterocyclic Compounds publishes original papers, short communications, reviews, and mini-reviews dealing with problems in the field of heterocyclic chemistry in Russian and English. The Journal also publishes reviews and annotations on new books and brief reports on conferences in the field of heterocyclic chemistry, as well as commemoratives dedicated to prominent heterocyclic chemists.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


