气液管式连续流钯催化氨基羰基化工艺用于羧酰胺的规模化合成
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
在此,我们介绍了一种高效的连续流工艺,该工艺集成了一个定制设计的气体输入控制系统,并与一个管式反应器相结合,用于钯催化的氨基羰基化反应,能够以碘烯烃和碘炔烃为底物,以一氧化碳为羰基源,以生物相关胺为亲核剂,生产多克级的羧酰胺。在中等反应条件下(压力≤ 3 bar,温度≤ 100 °C),我们合成了一系列单羧酰胺类化合物,即类固醇、吲哚和吡啶衍生物(包括一种拉扎贝胺类似物),化学选择性显著,生产率高达每天 21 克。本文章由计算机程序翻译,如有差异,请以英文原文为准。
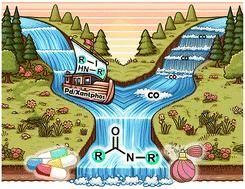
Gas–liquid tubular continuous-flow Pd-catalysed aminocarbonylation process for scalable synthesis of carboxamides†
Herein we describe an efficient continuous flow process that integrates a custom-designed gas input control system, coupled with a tubular reactor for Pd-catalysed aminocarbonylation, which is able to produce carboxamides in multigram scale, using iodoalkenes and iodoarenes as substrates, CO as the carbonyl source and biologically relevant amines as nucleophiles. Under moderate reaction conditions (pressure ≤ 3 bar and temperature ≤ 100 °C), we synthesised an array of monocarboxamides, namely steroids, indole, and pyridine derivatives (including a lazabemide analogue) with remarkable chemoselectivity, achieving a noteworthy productivity of up to 21 g per day.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


