同时改性富 Na 和 Ca2+/Ni2+ 双取代,提升 Na3V2(PO4)3 的卓越电化学性能
IF 8.6
2区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
由于 NaV(PO)(NVP)的本征电子电导率较低,严重限制了其进一步发展。本文提出了钙镍共掺杂和碳纳米管(CNTs)包覆的 NaVCaNi(PO)/C@CNTs(CaNi0.07@CNTs)体系。钙和镍都被 V 取代,引发电荷补偿并产生 p 型掺杂效应,产生大量空穴载流子,从而提高电子导电性。此外,Ca 的离子半径明显大于 V,因此引入 Ca 可以支撑 NVP 晶体结构并提高其稳定性。此外,Ca 的引入还能增加晶格间距,从而扩大钠离子的传输通道。镍的引入可减少电荷传输过程中的阻力,优化化学特性。同时,由于钙和镍的价数较低,为了平衡电荷,NVP 系统需要引入更多的 Na。富含 Na 的策略促使过量的活性 Na 参与去钙化过程,从而提供更多的可逆容量。此外,包裹在活性晶粒周围的 CNT 可缓冲晶体的变形,并建立连接颗粒的导电网络。循环后的 XRD/SEM/XPS 进一步证实了 CaNi0.07@CNTs 晶体稳定性的提高。综合来看,CaNi0.07@CNTs 在半电池和全电池中都具有优异的钠存储能力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
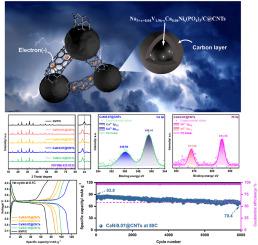
Simultaneous modification of Na-rich and Ca2+/Ni2+ dual-substitution boosting superior electrochemical performance of Na3V2(PO4)3
The lower intrinsic electronic conductivity of NaV(PO)(NVP) has seriously limited its further development. Herein, Ca/Ni co-doped and carbon nanotubes (CNTs)-coated NaVCaNi(PO)/C@CNTs (CaNi0.07@CNTs) system is presented. Both Ca and Ni are substituted for V, triggering charge compensation and producing p-type doping effect, generating abundant hole carriers to improve electronic conductivity. Furthermore, the ionic radius of Ca is significantly larger than that of V, so introduction of Ca can support NVP crystal structure and improve the stability. Furthermore, the introduction of Ca can increase the lattice spacing, thus expanding the transport channels for sodium ions. The introduction of Ni reduces the resistance suffered during charge transport and optimizes the chemical properties. Meanwhile, due to low valence of Ca and Ni, more Na are designed to be introduced to the NVP system for charge balance. The Na-rich strategy induces excess active Na participating in the de-intercalation process to supply more reversible capacities. Furthermore, the CNTs wrapped around the active grains serves to buffer deformation of the crystal and to establish a conductive network connecting the particles. The after-cycling XRD/SEM/XPS further confirms the improved crystal stability of CaNi0.07@CNTs. Comprehensively, CaNi0.07@CNTs possess superior sodium storage in half and full cells.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today Energy
Materials Science-Materials Science (miscellaneous)
CiteScore
15.10
自引率
7.50%
发文量
291
审稿时长
15 days
期刊介绍:
Materials Today Energy is a multi-disciplinary, rapid-publication journal focused on all aspects of materials for energy.
Materials Today Energy provides a forum for the discussion of high quality research that is helping define the inclusive, growing field of energy materials.
Part of the Materials Today family, Materials Today Energy offers authors rigorous peer review, rapid decisions, and high visibility. The editors welcome comprehensive articles, short communications and reviews on both theoretical and experimental work in relation to energy harvesting, conversion, storage and distribution, on topics including but not limited to:
-Solar energy conversion
-Hydrogen generation
-Photocatalysis
-Thermoelectric materials and devices
-Materials for nuclear energy applications
-Materials for Energy Storage
-Environment protection
-Sustainable and green materials
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


