Ni(OTf)2 催化的 4-羟基香豆素与 α、β-不饱和 2-酰基咪唑的迈克尔加成反应
IF 1.7
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在作为路易斯酸的 Ni(OTf)2 催化下,研究人员开发了一种将 4-羟基香豆素与 α、β-不饱和 2-酰基咪唑进行高效迈克尔加成的方法。在温和的条件下,催化剂负载量为 2 摩尔%时,可获得一系列 4-羟基香豆素衍生物,收率极高(高达 96%)。此外,当使用手性金属铑络合物作为催化剂时,还观察到了适度的对映体选择性(74% ee)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
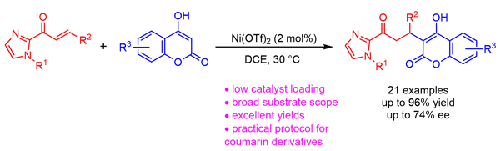
Ni(OTf)2-Catalyzed Michael Addition Reactions of 4-Hydroxycoumarins to α,β-Unsaturated 2-Acyl Imidazoles
An efficient Michael addition of 4-hydroxycoumarins to α,β-unsaturated 2-acyl imidazoles catalyzed by Ni(OTf)2 as a Lewis acid has been developed. A series of 4-hydroxycoumarin derivatives were obtained in excellent yields (up to 96%) with a 2 mol% catalyst loading under mild conditions. Additionally, when a chiral-at-metal rhodium complex was used as the catalyst, moderate enantioselectivity was observed (74% ee).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Synlett
化学-有机化学
CiteScore
3.40
自引率
5.00%
发文量
369
审稿时长
1 months
期刊介绍:
SYNLETT is an international journal reporting research results and current trends in chemical synthesis in short personalized reviews and preliminary communications. It covers all fields of scientific endeavor that involve organic synthesis, including catalysis, organometallic, medicinal, biological, and photochemistry, but also related disciplines and offers the possibility to publish scientific primary data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


