不对称催化中的 1,2-反式二氨基环己烷 (DACH):将近五十年的忠实服务和计数
摘要
本综述重点介绍了 DACH 作为多功能配体在催化不对称转化中的应用,按时间顺序介绍了 21 种反应类型中每种反应的机理依据和相关评论,并提供了截至 2023 年 12 月 25 日的参考文献。本综述旨在尽可能全面地介绍在不对称转化中使用 DACH 作为有机催化配体或金属配合物的有用实例。由此产生的对映体富集(即使不是纯的)手性非气相小分子作为天然产物全合成的增值中间体、具有重要药用价值的化合物的设计和合成以及手性发挥作用的有机和生物有机化学的其他领域,具有极大的实用价值。图中的斯巴达克斯双手合十,与 (R,R)-DACH 的手性意义相同。3 使用 DACH Ni(II) 复合物作为催化剂 6.4 使用 DACH H 键催化 7 烯烃的催化不对称环氧化 8 催化不对称克莱森重排 9 1,2-亲核羰基化合物的催化不对称加成 9.1 二烷基锌与醛和酮的催化不对称加成 9.2 醛和酮的催化不对称炔化 9.3 氰化物与醛和酮的催化不对称加成 10 催化不对称烯丙基烷基化 11 催化不对称烯环丙烷化 12 催化不对称环加成反应 13烯烃的催化不对称氮丙啶化反应 14 亲手性酮类和胺类的催化不对称加氢反应 15 催化不对称醛醇反应 16 小环系统的催化不对称开环 16.1 中环氧化物和中氮杂环丁烷的非对称化 16.2 外消旋环氧化物的动力学解析 16.3 CO2 与环氧化物的对映选择性加成 16.4 氧杂环丁烷的对映选择性开环 17 催化不对称 Strecker 反应 18 催化不对称 Mannich 反应 19 催化不对称 Henry 和 Aza-Henry 反应 20 催化不对称 Morita-Baylis-Hillman 和 Rauhut-Currier 反应 21 催化不对称 Petasis 反应 22 有机催化不对称级联反应 23 其它催化反应 24 结论与展望 25 DACH 催化剂和配体列表
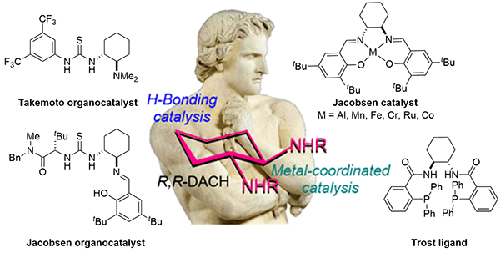
This review highlights the use of DACH as a versatile ligand in catalytic asymmetric transformations providing mechanistic rationales and relevant comments presented in chronological order for each of the 21 reaction types with references up to December 25, 2023. Intended to be as practically comprehensive as possible, this review assembles useful examples of using DACH as a ligand in organocatalytic or as metal complexes in asymmetric transformations. The resulting enantiomerically enriched, if not pure, chiral non-racemic small molecules are of great utility as value added intermediates in the total synthesis of natural products, in the design and synthesis of medicinally important compounds, and in other areas in organic and bioorganic chemistry where chirality plays a role. The graphic image depicts Spartacus with his arms folded in the same sense of chirality as (R,R)-DACH.
1 Introduction
2 DACH: A Brief Historical Narrative
3 Catalytic Asymmetric Hydrogenation of Alkenes
4 Catalytic Asymmetric Dihydroxylation of Alkenes
5 Catalytic Asymmetric Sulfoxidation and Sulfimidation
6 Catalytic Asymmetric 1,4-Conjugate Addition
6.1 Using Jacobsen’s DACH Metal–salen Complexes as Catalysts
6.2 Using Takemoto’s Bifunctional H-Bonding DACH Thiourea Organocatalyst
6.3 Using DACH Ni(II) Complexes as Catalysts
6.4 Using DACH H-Bonding Catalysis
7 Catalytic Asymmetric Epoxidation of Alkenes
8 Catalytic Asymmetric Claisen Rearrangement
9 Catalytic Asymmetric 1,2-Nucleophilic Addition to Carbonyl Compounds
9.1 Catalytic Asymmetric Addition of Dialkylzinc to Aldehydes and Ketones
9.2 Catalytic Asymmetric Alkynylation of Aldehydes and Ketones
9.3 Catalytic Asymmetric Addition of Cyanide to Aldehydes and Ketones
10 Catalytic Asymmetric Allylic Alkylation
11 Catalytic Asymmetric Cyclopropanation of Alkenes
12 Catalytic Asymmetric Cycloaddition Reactions
13 Catalytic Asymmetric Aziridination of Alkenes
14 Catalytic Asymmetric Hydrogenation of Prochiral Ketones and Imines
15 Catalytic Asymmetric Aldol Reactions
16 Catalytic Asymmetric Opening of Small Ring Systems
16.1 Desymmetrization of meso-Epoxides and meso-Aziridines
16.2 Kinetic Resolution of Racemic Epoxides
16.3 Enantioselective Addition of CO2 to Epoxides
16.4 Enantioselective Ring Opening of Oxetanes
17 Catalytic Asymmetric Strecker Reactions
18 Catalytic Asymmetric Mannich Reactions
19 Catalytic Asymmetric Henry and Aza-Henry Reactions
20 Catalytic Asymmetric Morita–Baylis–Hillman and Rauhut–Currier Reactions
21 Catalytic Asymmetric Petasis Reactions
22 Organocatalytic Asymmetric Cascade Reactions
23 Miscellaneous Catalytic Reactions
24 Conclusion and Outlook
25 DACH Catalysts and Ligands List

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


