通过原位生成的二氟烯醇中间体的迈克尔加成进行不对称二氟烷基化反应
IF 4.6
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本研究公开了一种二铑和手性 Zn 复合物共同催化的异亚甲基丙二腈的不对称二氟烷基化反应,该反应是通过迈克尔型截取 α,α-二氟烯醇物种实现的,α,α-二氟烯醇物种是在 Rh2(esp)2 存在下由三氟甲基重氮化合物和水原位生成的。该反应为合成含有手性季碳中心的氟化吲哚提供了一种有效的方法,而且产量普遍较高,立体选择性极佳(91%-99% ee)。与二氟烯氧基硅烷相比,这种二氟烯醇中间体在温和条件下具有更高的反应活性和对映选择性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
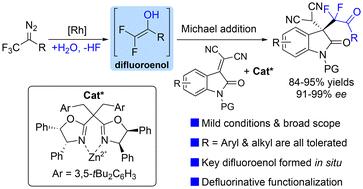
Asymmetric difluoroalkylation via Michael addition of an in situ generated difluoroenol intermediate†
A dirhodium and chiral Zn-complex co-catalyzed asymmetric difluoroalkylation of isatylidene malononitriles via a Michael-type interception of α,α-difluoroenol species, which are generated in situ from trifluoromethyl diazo compounds and water in the presence of Rh2(esp)2, has been disclosed. This reaction provides an efficient approach for the synthesis of fluorinated oxindoles containing a chiral quaternary carbon center in generally good yields and with excellent stereoselectivities (91%–99% ee). Compared with difluoroenoxysilane, this difluoroenol intermediate showed higher reactivity and enantioselectivity under mild conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Chemistry Frontiers
CHEMISTRY, ORGANIC-
CiteScore
7.90
自引率
11.10%
发文量
686
审稿时长
1 months
期刊介绍:
Organic Chemistry Frontiers is an esteemed journal that publishes high-quality research across the field of organic chemistry. It places a significant emphasis on studies that contribute substantially to the field by introducing new or significantly improved protocols and methodologies. The journal covers a wide array of topics which include, but are not limited to, organic synthesis, the development of synthetic methodologies, catalysis, natural products, functional organic materials, supramolecular and macromolecular chemistry, as well as physical and computational organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


