通过自由基-自由基交叉偶联实现光氧化催化的三组分烯碳三氟甲基化反应
IF 4.7
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在本研究中,我们建立了一种高效的光催化策略,用于烯烃的三组分三氟甲基化。这种方法能够在可见光照射下构建多种手性含碳 1,4-双(三氟甲基化)化合物。通过使用市场上可买到的 Togni 和 Langlois 试剂,在温和的条件下,无需额外添加剂,反应便可通过光氧化催化的自由基-自由基交叉偶联顺利进行。该工艺可用于克级规模的产品合成和生物活性分子的后期功能化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
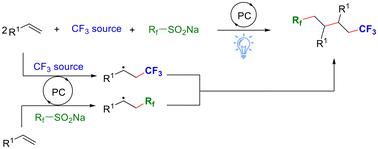
Photoredox-catalyzed three-component carbotrifluoromethylation of alkenes via radical–radical cross-coupling†
In this study, we establish an efficient photocatalytic strategy for three-component carbotrifluoromethylation of alkenes. This approach is capable of constructing a variety of chiral carbon-containing 1,4-bis(trifluoromethylated) compounds under visible light irradiation. By using commercially available Togni and Langlois reagents, the reaction can smoothly proceed via photoredox-catalyzed radical–radical cross-coupling in the absence of additional additives under mild conditions. Application of the process in gram-scale synthesis of the product and late-stage functionalization of bioactive molecules is achieved.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Chemistry Frontiers
CHEMISTRY, ORGANIC-
CiteScore
7.90
自引率
11.10%
发文量
686
审稿时长
1 months
期刊介绍:
Organic Chemistry Frontiers is an esteemed journal that publishes high-quality research across the field of organic chemistry. It places a significant emphasis on studies that contribute substantially to the field by introducing new or significantly improved protocols and methodologies. The journal covers a wide array of topics which include, but are not limited to, organic synthesis, the development of synthetic methodologies, catalysis, natural products, functional organic materials, supramolecular and macromolecular chemistry, as well as physical and computational organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


