上调的 SAE1 驱动肿瘤发生并与乳腺癌的不良临床预后有关
摘要
研究背景本研究旨在分析 SUMO 激活酶亚基 1(SAE1)在乳腺癌(BC)中的表达。通过生物信息学分析和体外实验,进一步分析 SAE1 在 BC 中的生物学功能和可能相关的信号通路。研究方法应用生物信息学分析方法分析 SAE1 在 BC 和正常乳腺组织中的表达、其与 BC 患者临床病理特征和预后的关系,以及癌症基因组图谱数据库和基因表达总集数据集的数据。我们用免疫组化方法分析了79例乳腺癌患者的BC组织和癌旁组织中SAE1的表达情况。用细胞计数试剂盒-8 和集落形成试验检测 BC 细胞的增殖情况。流式细胞术分析了细胞周期的进展,Western印迹测定了SAE1小干扰RNA(siRNA)转染细胞中细胞周期相关蛋白(E2F1、细胞周期蛋白D3和细胞周期蛋白依赖性激酶2)的表达。利用基因组富集分析(GSE1456)数据集分析与SAE1相关的可能信号通路,并通过Western印迹检测PI3K/AKT/mTOR通路相关蛋白(如p-PI3K、p-AKT和mTOR)在SAE1-siRNA细胞中的表达。结果生物信息学和免疫组化结果显示,SAE1 mRNA和蛋白在BC组织中的表达量明显高于正常组织。SAE1的过表达与肿瘤大小、肿瘤-结节-转移分期、雌激素受体、孕激素受体、人表皮生长因子受体2以及是否为三阴性BC明显相关。与低表达患者相比,SAE1过表达患者的总生存期(OS)、无复发生存期(RFS)和无远处转移生存期均较差。多变量Cox回归分析表明,SAE1可能是影响BC患者OS的一个独立预后因素。SAE1-siRNA在体外抑制了BC细胞的增殖和细胞周期过程。GSEA结果显示,SAE1与12个基因组显著相关,包括未折叠蛋白反应、DNA修复、氧化磷酸化和细胞周期等。此外,mTORC1和PI3K/Akt/mTOR这两个信号通路与SAE1的过表达有明显相关性。Western 印迹证实,在敲除 SAE1 后,PI3K/Akt/mTOR 通路相关蛋白(p-PI3K、p-AKT 和 mTOR)在 BC 细胞中的表达量减少。结论SAE1在BC细胞中高表达。SAE1的过表达与BC的不良预后有关。此外,它还是 BC 患者的一个独立预后因素。我们证实,体外敲除 SAE1 能有效抑制 BC 增殖及其细胞周期过程。此外,SAE1的生物学功能可能与PI3K/Akt/mTOR通路有关。SAE1将成为治疗BC的潜在靶点。
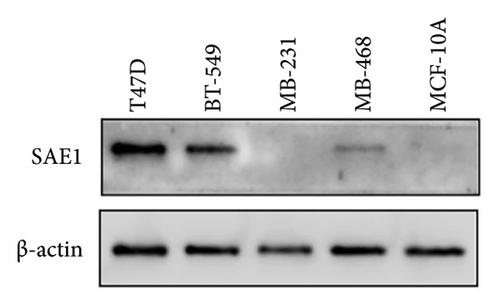
Background. The purpose of this study was to analyze SUMO activating enzyme subunit 1 (SAE1) expression in breast cancer (BC). Through bioinformatics analysis and in vitro experiments, the biological function and possibly associated signal pathways of SAE1 in BC were further analyzed. Methods. Bioinformatics analysis was applied to analyze SAE1 expression in BC and normal breast tissues, its relationship with clinicopathologic characteristics and prognosis in BC patients, and data from the Cancer Genome Atlas database and Gene Expression Omnibus dataset. We performed immunohistochemistry to analyze SAE1 expression in BC tissues and para-cancer tissues in 79 breast cancer patients. BC cell proliferation was detected with the Cell Counting Kit-8 and by the colony formation assay. Cell cycle progression was analyzed by flow cytometry, and the expression of cell cycle-related proteins (E2F1, cyclin D3, and cyclin-dependent kinase 2) was determined by western blots in SAE1 small interfering RNA (siRNA) transfected cells. The GSE1456 dataset was used to analyze possible signal pathways associated with SAE1 by gene set enrichment analysis (GSEA), and the expression of PI3K/AKT/mTOR pathway-related proteins (such as p-PI3K, p-AKT, and mTOR) in SAE1-siRNA cells was detected by western blots. Results. The bioinformatics and immunohistochemical results showed that SAE1 mRNA and protein expression in BC tissues were significantly higher than those in normal tissues. The SAE1 overexpression was significantly associated with the tumor size, tumor-node-metastasis stage, estrogen receptor, progesterone receptor, human epidermal growth factor receptor 2, and whether or not it was a triple-negative BC. Patients with SAE1 overexpression had a worse overall survival (OS), recurrence-free survival (RFS), and distant metastasis-free survival compared with lower expression patients. Multivariate Cox regression analysis showed that SAE1 may be an independent prognostic factor for OS of BC patients. The proliferation and cell cycle process of BC cells were inhibited by SAE1-siRNA in vitro. The result of GSEA showed that SAE1 was significantly associated with 12 gene sets, including unfolded protein reaction, DNA repair, oxidative phosphorylation, and cell cycle, among others. Additionally, two signal pathways, mTORC1 and PI3K/Akt/mTOR, were significantly correlated with SAE1 overexpression. Western blots confirmed that the expression of PI3K/Akt/mTOR pathway-related proteins (p-PI3K, p-AKT, and mTOR) in BC cells was decreased after knocking down SAE1. Conclusion. SAE1 was highly expressed in BC. Its overexpression was associated with poor BC prognosis. Additionally, it was an independent prognostic factor for BC patients. We demonstrated that in vitro SAE1 knockdown effectively inhibited BC proliferation and its cell cycle process. Furthermore, the biological function of SAE1 may be associated with the PI3K/Akt/mTOR pathway. SAE1 will be a potential target for BC treatment.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


