作为逆转录酶抑制剂的 2′-β-甲基硒核苷(t)ide 类似物可抑制多种艾滋病毒突变体
IF 3.3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
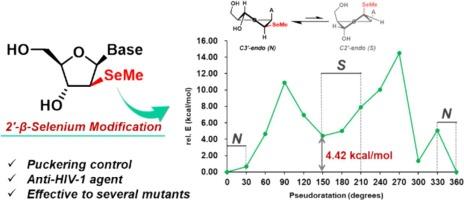
核苷类逆转录酶抑制剂(NRTIs)作为针对 HIV RT 的药物已被广泛研究。然而,实践或使用已批准的缺乏3ʹ-羟基的NRTIs往往会导致HIV频繁突变并产生耐药性。在这里,我们描述了一种具有 2′-β-甲基硒基修饰的新型 NRTI。我们发现,尽管这种修饰具有 3′-羟基,但它能抑制 HIV-1 RT 的 DNA 延长反应。此外,这种核苷类似物的构象被控制在 C3′-内端,这种构象能阻止 HIV RT 从伸长的 DNA 中切除。因此,所设计的类似物对野生型 HIV 和具有多重耐药性的 HIV 突变体都具有活性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
2′-β-Methylselenyl nucleos(t)ide analogs as reverse transcriptase inhibitors against diverse HIV mutants
Nucleoside reverse transcriptase inhibitors (NRTIs) have been extensively studied as drugs targeting HIV RT. However, the practice or use of approved NRTIs lacking the 3ʹ-hydroxy group often promotes frequent HIV mutations and generates drug-resistance. Here, we describe a novel NRTI with 2′-β-methylselenyl modification. We found that this modification inhibited the DNA elongation reaction by HIV-1 RT despite having a 3′-hydroxy group. Moreover, the conformation of this nucleoside analog is controlled at C3′-endo, a conformation that resists excision from the elongating DNA by HIV RT. Accordingly, the designed analogs exhibited activity against both wild-type HIV and multidrug-resistant HIV mutants.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


