揭示蠕虫病理学中的新角色:来自肝脏法氏囊和树枝状微囊藻的细胞外囊泡对肝星状细胞和肝细胞产生不同影响。
IF 3.7
2区 医学
Q1 PARASITOLOGY
引用次数: 0
摘要
Fasciola hepatica 和 Dicrocoelium dendriticum 是寄生在哺乳动物宿主胆管中的吸虫,在某些情况下会导致肝功能受损和肝纤维化。以前的研究表明,肝吸虫(FhEVs)和树枝状吸虫(DdEVs)释放的胞外囊泡会诱导人类巨噬细胞出现不同的表型,但关于寄生EVs对肝细胞影响的信息却很有限,因为在自然感染中,肝细胞会直接与虫体发生相互作用。在这项研究中,我们通过尺寸排阻色谱法分离了FhEVs和DdEVs,并用亲脂性荧光染料对其进行标记,以合成脂质体作为内部标记和摄取对照,分析肝脏病理学中重要的细胞类型--人肝星状细胞(HSC)和肝细胞对它们的摄取情况。我们分析了这两种细胞对EVs的吸收情况以及EVs处理后的蛋白质组图谱。我们的研究结果表明,EVs 与星状细胞和肝细胞建立了独特的特异性相互作用,这表明每种寄生虫产生的 EVs 都有不同的作用,这取决于它们到达最终龛位的迁移路线。FhEVs对造血干细胞有细胞抑制作用,但能诱导细胞外基质分泌并引起肝细胞的抗炎反应。与 FhEVs 相比,DdEVs 具有更强的抗增殖作用,并引发整体炎症反应,提高两种细胞中 NF-κB 和其他炎症介质的水平。这些相互作用可能会对疾病的进展产生重大影响,从而产生有利于蠕虫在宿主体内繁殖的条件。本文章由计算机程序翻译,如有差异,请以英文原文为准。
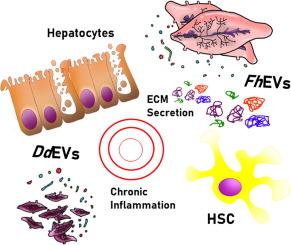
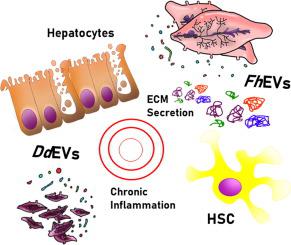
Unraveling new players in helminth pathology: extracellular vesicles from Fasciola hepatica and Dicrocoelium dendriticum exert different effects on hepatic stellate cells and hepatocytes
Fasciola hepatica and Dicrocoelium dendriticum are parasitic trematodes residing in the bile ducts of mammalian hosts, causing, in some cases, impairment of liver function and hepatic fibrosis. Previous studies have shown that extracellular vesicles released by F. hepatica (FhEVs) and D. dendriticum (DdEVs) induce a distinct phenotype in human macrophages, but there is limited information on the effect of parasitic EVs on liver cells, which interact directly with the worms in natural infections. In this study, we isolated FhEVs and DdEVs by size exclusion chromatography and labeled them with a lipophilic fluorescent dye to analyze their uptake by human hepatic stellate cells (HSC) and hepatocytes, important cell types in liver pathology, using synthetic liposomes as internal labeling and uptake control. We analyzed EV uptake and the proteome profiles after the treatment with EVs for both cell types. Our results reveal that EVs establish unique and specific interactions with stellate cells and hepatocytes, suggesting a different role of EVs derived from each parasite, depending on the migration route to reach their final niche. FhEVs have a cytostatic effect on HSCs, but induce the extracellular matrix secretion and elicit anti-inflammatory responses in hepatocytes. DdEVs have a more potent anti-proliferative effect than FhEVs and trigger a global inflammatory response, increasing the levels of NF-κB and other inflammatory mediators in both cell types. These interactions may have a major influence on the progression of the disease, serving to generate conditions that may favor the establishment of the helminths in the host.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.40
自引率
2.50%
发文量
76
审稿时长
23 days
期刊介绍:
International Journal for Parasitology offers authors the option to sponsor nonsubscriber access to their articles on Elsevier electronic publishing platforms. For more information please view our Sponsored Articles page. The International Journal for Parasitology publishes the results of original research in all aspects of basic and applied parasitology, including all the fields covered by its Specialist Editors, and ranging from parasites and host-parasite relationships of intrinsic biological interest to those of social and economic importance in human and veterinary medicine and agriculture.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


