在原位胶质母细胞瘤小鼠模型中通过靶向 COUP-TFII 抗血管生成和抗免疫抑制基因疗法。
IF 4.8
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
摘要
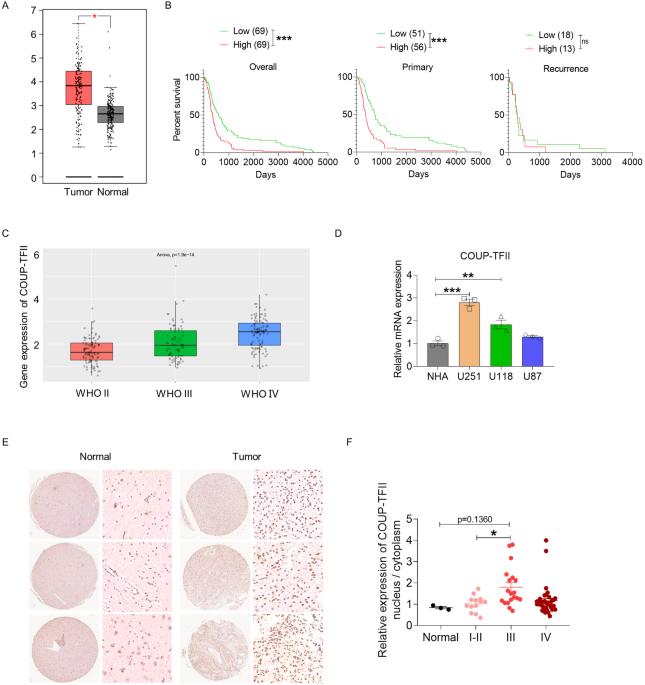
胶质母细胞瘤(GBM)是最常见、最具侵袭性的原发性脑癌;血管生成和免疫抑制加剧了 GBM 的进展。COUP-TFII 具有促进血管生成的活性,但其在胶质瘤进展中的作用仍不清楚。本研究发现,COUP-TFII 通过诱导胶质瘤细胞向内皮样细胞的转分化,促进胶质瘤的血管生成。机理研究表明,COUP-TFII作为一种转录因子,是通过与TXNIP的启动子结合来发挥其功能的。有趣的是,在免疫功能正常的小鼠模型中,COUP-TFII的敲除可减轻肿瘤发生和肿瘤进展,但在免疫缺陷小鼠模型中,COUP-TFII却可促进肿瘤进展。这可能是因为 COUP-TFII 的抑制诱导了细胞衰老,激活了胶质瘤细胞在体外和体内的免疫监视。此外,我们使用肝素-聚乙烯亚胺(HPEI)纳米颗粒递送 COUP-TFII shRNA,在原位 GBM 小鼠模型中调节肿瘤血管生成和免疫抑制。这项研究为治疗 GBM 提供了一种新策略和潜在的治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Anti-angiogenesis and anti-immunosuppression gene therapy through targeting COUP-TFII in an in situ glioblastoma mouse model
Glioblastoma (GBM) is the most common and aggressive primary brain cancer; angiogenesis and immunosuppression exacerbate GBM progression. COUP-TFII demonstrates pro-angiogenesis activity; however, its role in glioma progression remains unclear. This study revealed that COUP-TFII promotes angiogenesis in gliomas by inducing transdifferentiation of glioma cells into endothelial-like cells. Mechanistic investigation suggested that COUP-TFII as a transcription factor exerts its function via binding to the promoter of TXNIP. Interestingly, COUP-TFII knockdown attenuated tumorigenesis and tumor progression in an immunocompetent mouse model but promoted tumor progression in an immuno-deficient mouse model. As an explanation, repression of COUP-TFII induces cellular senescence and activates immune surveillance in glioma cells in vitro and in vivo. In addition, we used heparin–polyethyleneimine (HPEI) nanoparticles to deliver COUP-TFII shRNA, which regulated tumor angiogenesis and immunosuppression in an in situ GBM mouse model. This study provides a novel strategy and potential therapeutic targets to treat GBM.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


