通过定时进餐瞄准肠道昼夜节律钟可改善胃肠道炎症。
IF 21.8
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
据观察,在炎症性肠病(IBD)患者的活组织切片中,时钟基因的表达受到了影响。倒班工人的昼夜节律紊乱与胃肠道疾病(包括 IBD)的患病风险增加有关。肠上皮细胞(IECs)中的外周昼夜节律钟以前曾被证明能通过调节微生物群来平衡胃肠道的平衡。在这里,我们证明了肠时钟在与 IBD 相关的小鼠模型(IL-10-/-)中被破坏。在化学和新型遗传诱导的结肠炎模型(DSS,Bmal1IEC-/-xIL-10-/-)中,肠上皮细胞(IECs)缺乏肠道时钟基因(Bmal1)会促进结肠炎的发生,并显著降低存活率。无芽胞 Bmal1IEC-/- 小鼠定植了 IL-10-/- 小鼠的疾病相关微生物群后,炎症反应加剧,这凸显了局部肠道时钟对微生物群诱导的 IBD 发展的重要性。在IL-10-/-小鼠中通过定时限制喂食(RF)直接靶向肠道时钟,可恢复肠道时钟功能,包括免疫细胞招募和微生物节律性;改善炎症反应;显著提高存活率并挽救组织病理学表型。相比之下,RF 未能改善 Bmal1IEC-/-xIL-10-/- 小鼠的 IBD 症状,这表明了肠道时钟在决定 RF 的有益作用方面的重要性。总之,我们提供的证据表明,肠道时钟功能失调会引发宿主免疫失衡,并促进类 IBD 结肠炎的发生和发展。通过射频增强肠道时钟功能可调节 IBD 的发病机制,因此可成为改善 IBD 患者症状的一种新策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
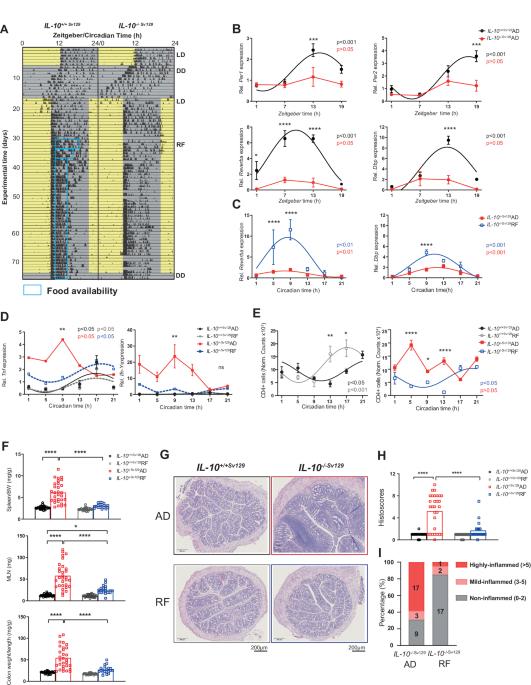
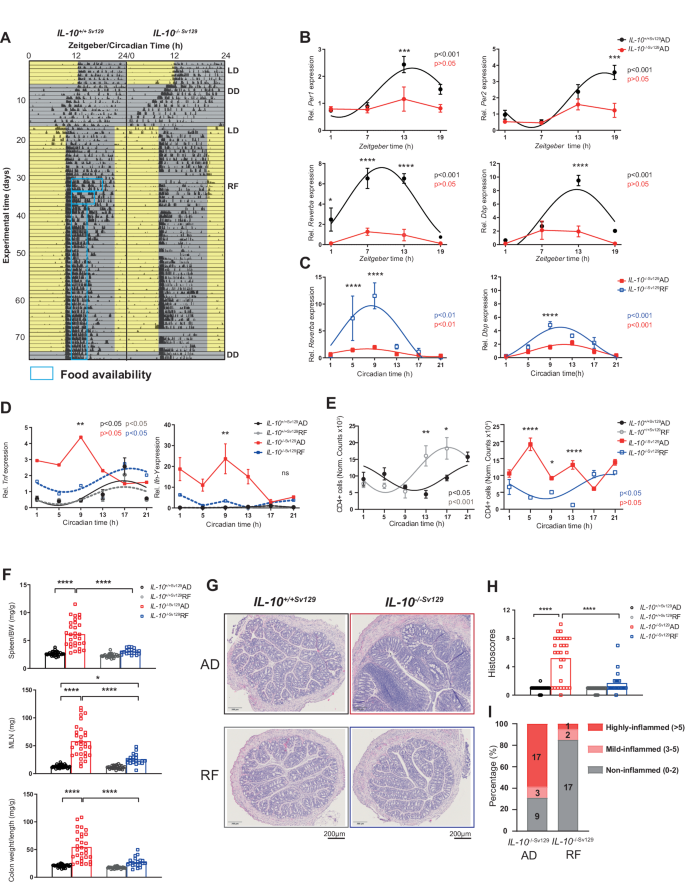
Targeting the intestinal circadian clock by meal timing ameliorates gastrointestinal inflammation
The expression of clock genes has been observed to be impaired in biopsies from patients with inflammatory bowel disease (IBD). Disruption of circadian rhythms, which occurs in shift workers, has been linked to an increased risk of gastrointestinal diseases, including IBD. The peripheral circadian clock in intestinal epithelial cells (IECs) was previously shown to balance gastrointestinal homeostasis by regulating the microbiome. Here, we demonstrated that the intestinal clock is disrupted in an IBD-relevant mouse model (IL-10−/−). A lack of the intestinal clock gene (Bmal1) in intestinal epithelial cells (IECs) in a chemically and a novel genetically induced colitis model (DSS, Bmal1IEC−/−xIL-10−/−) promoted colitis and dramatically reduced survival rates. Germ-free Bmal1IEC−/− mice colonized with disease-associated microbiota from IL-10−/− mice exhibited increased inflammatory responses, highlighting the importance of the local intestinal clock for microbiota-induced IBD development. Targeting the intestinal clock directly by timed restricted feeding (RF) in IL-10−/− mice restored intestinal clock functions, including immune cell recruitment and microbial rhythmicity; improved inflammatory responses; dramatically enhanced survival rates and rescued the histopathological phenotype. In contrast, RF failed to improve IBD symptoms in Bmal1IEC−/−xIL-10−/− mice, demonstrating the significance of the intestinal clock in determining the beneficial effect of RF. Overall, we provide evidence that intestinal clock dysfunction triggers host immune imbalance and promotes the development and progression of IBD-like colitis. Enhancing intestinal clock function by RF modulates the pathogenesis of IBD and thus could become a novel strategy to ameliorate symptoms in IBD patients.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
31.20
自引率
1.20%
发文量
903
审稿时长
1 months
期刊介绍:
Cellular & Molecular Immunology, a monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, serves as a comprehensive platform covering both basic immunology research and clinical applications. The journal publishes a variety of article types, including Articles, Review Articles, Mini Reviews, and Short Communications, focusing on diverse aspects of cellular and molecular immunology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


