E3泛素连接酶TRIM31通过促进帕金森病模型中VDAC1的蛋白酶体降解来缓解多巴胺能神经退行性病变
IF 13.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
线粒体功能障碍在帕金森病(PD)的发病机制中起着关键作用。作为线粒体调控因子,电压依赖性阴离子通道 1(VDAC1)对细胞存活和死亡信号至关重要,并与神经退行性疾病有关。然而,人们对 VDAC1 的调控机制知之甚少,而线粒体中富含的 E3 泛素连接酶--含三方基序蛋白 31(TRIM31)在帕金森病中的作用仍不清楚。在这项研究中,我们发现 TRIM31-/- 小鼠会自发出现与年龄相关的运动缺陷和多巴胺能(DA)神经变性。此外,TRIM31在MPTP诱导的PD小鼠黑质区域和MPP+刺激的SH-SY5Y细胞中均显著减少。TRIM31缺乏会明显加重MPTP诱导的DA神经毒性。从机理上讲,TRIM31与VDAC1相互作用,并通过其E3泛素连接酶活性催化K48连接的多泛素化,从而降解VDAC1。总之,我们首次证明了TRIM31通过泛素蛋白酶体途径促进VDAC1降解,从而成为DA神经元稳态的重要调节因子。我们的研究发现,TRIM31是一个新的潜在治疗靶点,对TRIM31和VDAC1之间的相互作用进行药物干预可能会为治疗帕金森病提供一种有前景的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。

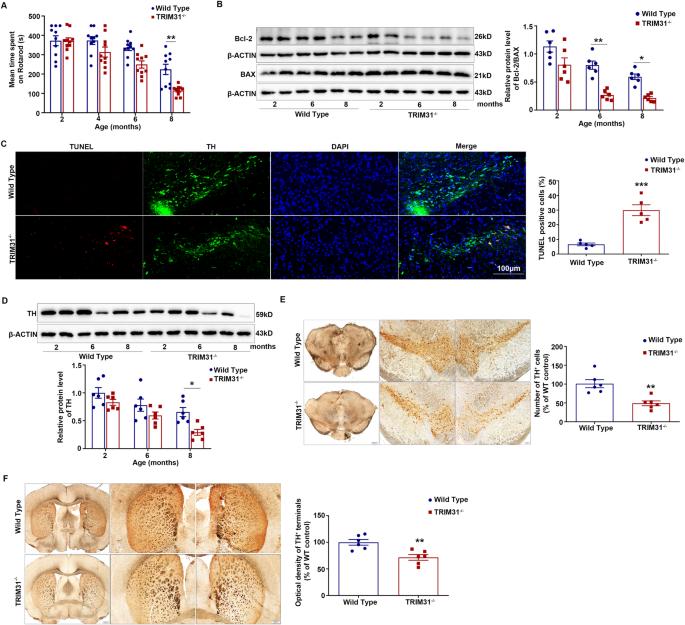
E3 ubiquitin ligase TRIM31 alleviates dopaminergic neurodegeneration by promoting proteasomal degradation of VDAC1 in Parkinson’s Disease model
Mitochondrial dysfunction plays a pivotal role in the pathogenesis of Parkinson’s disease (PD). As a mitochondrial governor, voltage-dependent anion channel 1 (VDAC1) is critical for cell survival and death signals and implicated in neurodegenerative diseases. However, the mechanisms of VDAC1 regulation are poorly understood and the role of tripartite motif-containing protein 31 (TRIM31), an E3 ubiquitin ligase which is enriched in mitochondria, in PD remains unclear. In this study, we found that TRIM31−/− mice developed age associated motor defects and dopaminergic (DA) neurodegeneration spontaneously. In addition, TRIM31 was markedly reduced both in nigrostriatal region of PD mice induced by MPTP and in SH-SY5Y cells stimulated by MPP+. TRIM31 deficiency significantly aggravated DA neurotoxicity induced by MPTP. Mechanistically, TRIM31 interacted with VDAC1 and catalyzed the K48-linked polyubiquitination to degrade it through its E3 ubiquitin ligase activity. In conclusion, we demonstrated for the first time that TRIM31 served as an important regulator in DA neuronal homeostasis by facilitating VDAC1 degradation through the ubiquitin-proteasome pathway. Our study identified TRIM31 as a novel potential therapeutic target and pharmaceutical intervention to the interaction between TRIM31 and VDAC1 may provide a promising strategy for PD.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell Death and Differentiation
生物-生化与分子生物学
CiteScore
24.70
自引率
1.60%
发文量
181
审稿时长
3 months
期刊介绍:
Mission, vision and values of Cell Death & Differentiation:
To devote itself to scientific excellence in the field of cell biology, molecular biology, and biochemistry of cell death and disease.
To provide a unified forum for scientists and clinical researchers
It is committed to the rapid publication of high quality original papers relating to these subjects, together with topical, usually solicited, reviews, meeting reports, editorial correspondence and occasional commentaries on controversial and scientifically informative issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


