给药顺序的疗效:萨妥珠单抗 Govitecan 和曲妥珠单抗 Deruxtecan 对 HER2 低的转移性乳腺癌的疗效。
IF 6.4
1区 医学
Q1 ONCOLOGY
引用次数: 0
摘要
背景:现行指南建议 HER2 低的转移性乳腺癌(MBC)患者依次接受两种抗体药物共轭物(ADC)治疗:萨妥珠单抗戈维替康(SG)和曲妥珠单抗德鲁司替康(T-DXd),尽管它们的有效载荷相似。然而,二者的疗效尚不清楚:ADC-Low是一项多中心回顾性研究,评估了HER2低下的MBC患者相继接受SG和T-DXd化疗或不接受中间化疗的疗效:结果:共纳入179例患者:大多数HR阴性肿瘤患者首先接受SG治疗(ADC1)(n=100/108),而大多数HR阳性肿瘤患者首先接受T-DXd治疗(n=56/71)。无进展生存期中位数2很短:全部患者为2.7个月(95% CI:2.4-3.3),接受T-DXd或SG治疗(ADC2)的患者分别为3.1个月(95% CI:2.6-3.6)和2.2个月(95% CI:1.9-2.7)。ADC1和ADC2之间的中间化疗线没有影响。54.4%的患者对ADC2产生了原发性耐药性。某些患者对ADC2产生了初步反应:结论:对大多数患者而言,连续使用 SG 和 T-DXd 的临床疗效有限。然而,一部分患者可能会在短期内从第二次 ADC 中获益。还需要进行更多研究,以确定哪些患者可以从两种有效载荷相似的 ADC 中获益。本文章由计算机程序翻译,如有差异,请以英文原文为准。
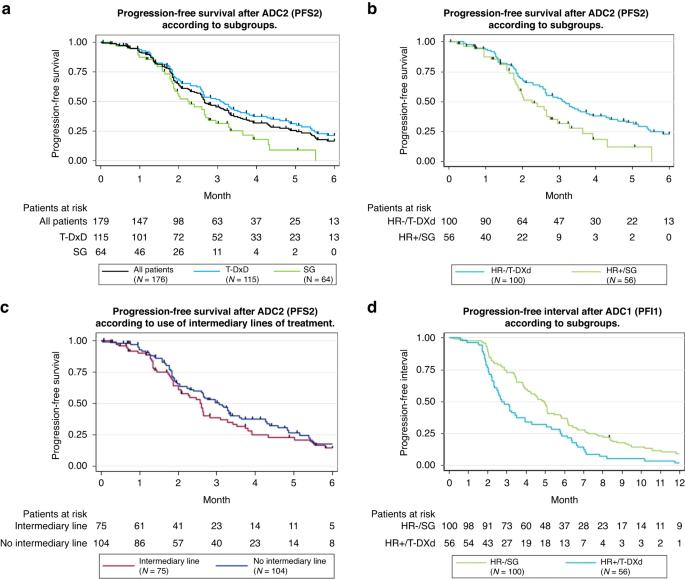
Efficacy of administration sequence: Sacituzumab Govitecan and Trastuzumab Deruxtecan in HER2-low metastatic breast cancer
Current guidelines recommend that patients with HER2-low metastatic breast cancer (MBC) receive sequentially two antibody–drug conjugates (ADCs): Sacituzumab Govitecan (SG) and Trastuzumab Deruxtecan (T-DXd), despite a similar payload. However, the effectiveness of one after another is unknown. ADC-Low is a multicentre, retrospective study evaluating the efficacy of SG and T-DXd, one after another, with or without intermediary lines of chemotherapy, in patients with HER2-low MBC. One hundred and seventy-nine patients were included: the majority with HR-negative tumours received SG first (ADC1) (n = 100/108) while most with HR-positive tumours received T-DXd first (n = 56/71). Median progression-free survival 2 was short: 2.7 months (95% CI: 2.4–3.3) in the whole population, respectively, 3.1 (95% CI: 2.6–3.6) and 2.2 months (95% CI: 1.9–2.7) for patients receiving T-DXd or SG second (ADC2). Intermediary lines of chemotherapy between ADC1 and ADC2 had no impact. Primary resistance to ADC2 occurred in 54.4% of patients. Certain patients showed initial response to ADC2. Clinical benefit of sequentially administered SG and T-DXd is limited for most patients. Nevertheless, a subset of patients might benefit—on the short term—from a second ADC. Additional studies are needed to identify patients who could benefit from two ADCs with similar payloads.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

British Journal of Cancer
医学-肿瘤学
CiteScore
15.10
自引率
1.10%
发文量
383
审稿时长
6 months
期刊介绍:
The British Journal of Cancer is one of the most-cited general cancer journals, publishing significant advances in translational and clinical cancer research.It also publishes high-quality reviews and thought-provoking comment on all aspects of cancer prevention,diagnosis and treatment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


