更新 RasGAP 蛋白 DAB2IP 的肿瘤抑制功能,重点关注其治疗意义。
IF 13.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
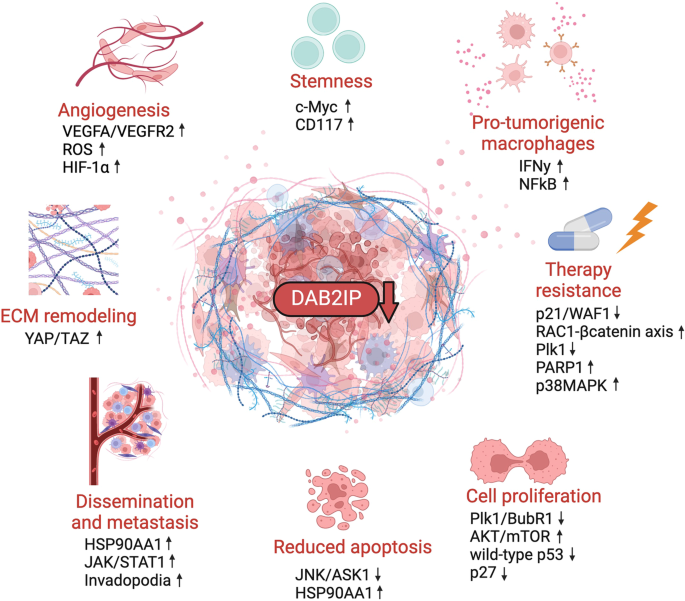
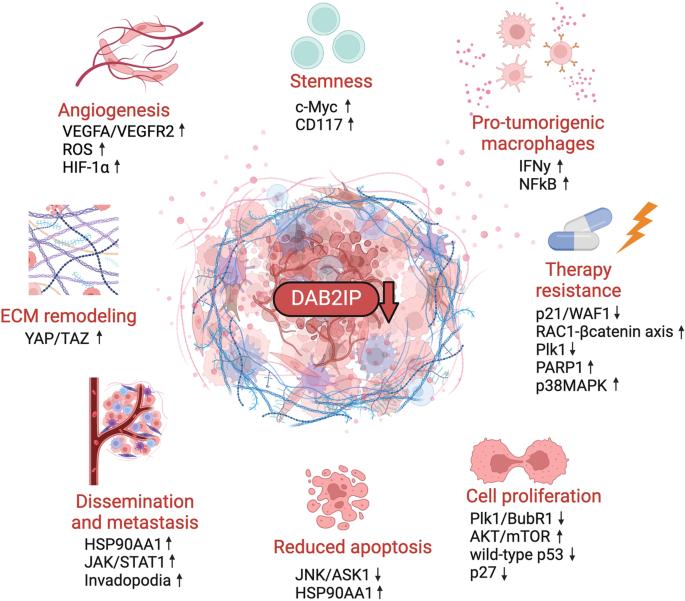
肿瘤和基质细胞之间的动态串扰是癌症侵袭性的主要决定因素。肿瘤抑制因子 DAB2IP(残障同源物 2 互作蛋白)在这种情况下发挥着重要作用,因为它能调节细胞对多种细胞外输入的反应,包括炎症细胞因子和生长因子。DAB2IP 是一种 RasGAP,对 Ras 依赖性有丝分裂信号起负性控制作用。此外,它还能调节其他主要致癌途径,包括 TNFα/NF-κB、WNT/β-catenin、PI3K/AKT 和雄激素受体信号传导。与 DAB2IP 的肿瘤抑制作用相一致的是,DAB2IP 在癌症中经常通过转录和转录后机制失活,包括启动子甲基化、microRNA 介导的下调以及蛋白质与蛋白质之间的相互作用。耐人寻味的是,一些观察结果表明,肿瘤基质细胞中 DAB2IP 的下调可能会促进有利于转移的微环境的建立。本综述总结了最近对 DAB2IP 的肿瘤抑制功能及其在癌症中失活的后果的见解。特别是,我们探讨了旨在重新激活 DAB2IP 或提高其表达水平的潜在方法,以此作为治疗癌症的新策略。我们认为,重新激活或上调 DAB2IP 将同时减弱癌细胞和肿瘤微环境中的多种致癌途径,这对改善多种肿瘤的治疗具有重要意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。
An update on the tumor-suppressive functions of the RasGAP protein DAB2IP with focus on therapeutic implications
The dynamic crosstalk between tumor and stromal cells is a major determinant of cancer aggressiveness. The tumor-suppressor DAB2IP (Disabled homolog 2 interacting protein) plays an important role in this context, since it modulates cell responses to multiple extracellular inputs, including inflammatory cytokines and growth factors. DAB2IP is a RasGAP and negatively controls Ras-dependent mitogenic signals. In addition, it modulates other major oncogenic pathways, including TNFα/NF-κB, WNT/β-catenin, PI3K/AKT, and androgen receptor signaling. In line with its tumor-suppressive role, DAB2IP is frequently inactivated in cancer by transcriptional and post-transcriptional mechanisms, including promoter methylation, microRNA-mediated downregulation, and protein-protein interactions. Intriguingly, some observations suggest that downregulation of DAB2IP in cells of the tumor stroma could foster establishment of a pro-metastatic microenvironment. This review summarizes recent insights into the tumor-suppressive functions of DAB2IP and the consequences of its inactivation in cancer. In particular, we explore potential approaches aimed at reactivating DAB2IP, or augmenting its expression levels, as a novel strategy in cancer treatment. We suggest that reactivation or upregulation of DAB2IP would concurrently attenuate multiple oncogenic pathways in both cancer cells and the tumor microenvironment, with implications for improved treatment of a broad spectrum of tumors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell Death and Differentiation
生物-生化与分子生物学
CiteScore
24.70
自引率
1.60%
发文量
181
审稿时长
3 months
期刊介绍:
Mission, vision and values of Cell Death & Differentiation:
To devote itself to scientific excellence in the field of cell biology, molecular biology, and biochemistry of cell death and disease.
To provide a unified forum for scientists and clinical researchers
It is committed to the rapid publication of high quality original papers relating to these subjects, together with topical, usually solicited, reviews, meeting reports, editorial correspondence and occasional commentaries on controversial and scientifically informative issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


