基于构象和激活的 BRET 传感器对 GPCR-G 蛋白耦合有不同的报告。
IF 6.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
G 蛋白偶联受体(GPCR)通过与由 Gα、Gβ 和 Gγ 亚基组成的异三聚体 G 蛋白的不同组合耦合来调节细胞信号传导过程。基于生物发光共振能量转移(BRET)的生物传感器推进了我们对 GPCR 功能选择性的了解。一些 BRET 生物传感器监测配体诱导的受体或 G 蛋白构象变化,而另一些则监测下游效应物招募到 G 蛋白活化位点的情况。在这里,我们比较了基于构象和活化的 BRET 生物传感器评估培养细胞中各种 A 类和 B 类 GPCR 与特定 Gα 蛋白耦合的能力。这些 GPCR 包括血清素 5-HT2A 和 5-HT7 受体、GLP-1 受体(GLP-1R)和 M3 肌卡因受体。我们观察到两类传感器的信号特征各不相同,这突出说明了数据解读可能会受到生物传感器性质的影响。我们还发现,检测中使用的 Gβγ 亚基的特性会不同程度地影响受体对 Gα 亚型的选择性,这强调了受体-Gβγ 配对在决定 Gα 偶联特异性方面的重要性。最后,在受体上添加表位标签可能会影响化学计量和耦合选择性,并产生伪造的结果。这些结果突出表明,在探测 GPCR-G 蛋白耦合时,需要仔细选择传感器和设计实验。本文章由计算机程序翻译,如有差异,请以英文原文为准。
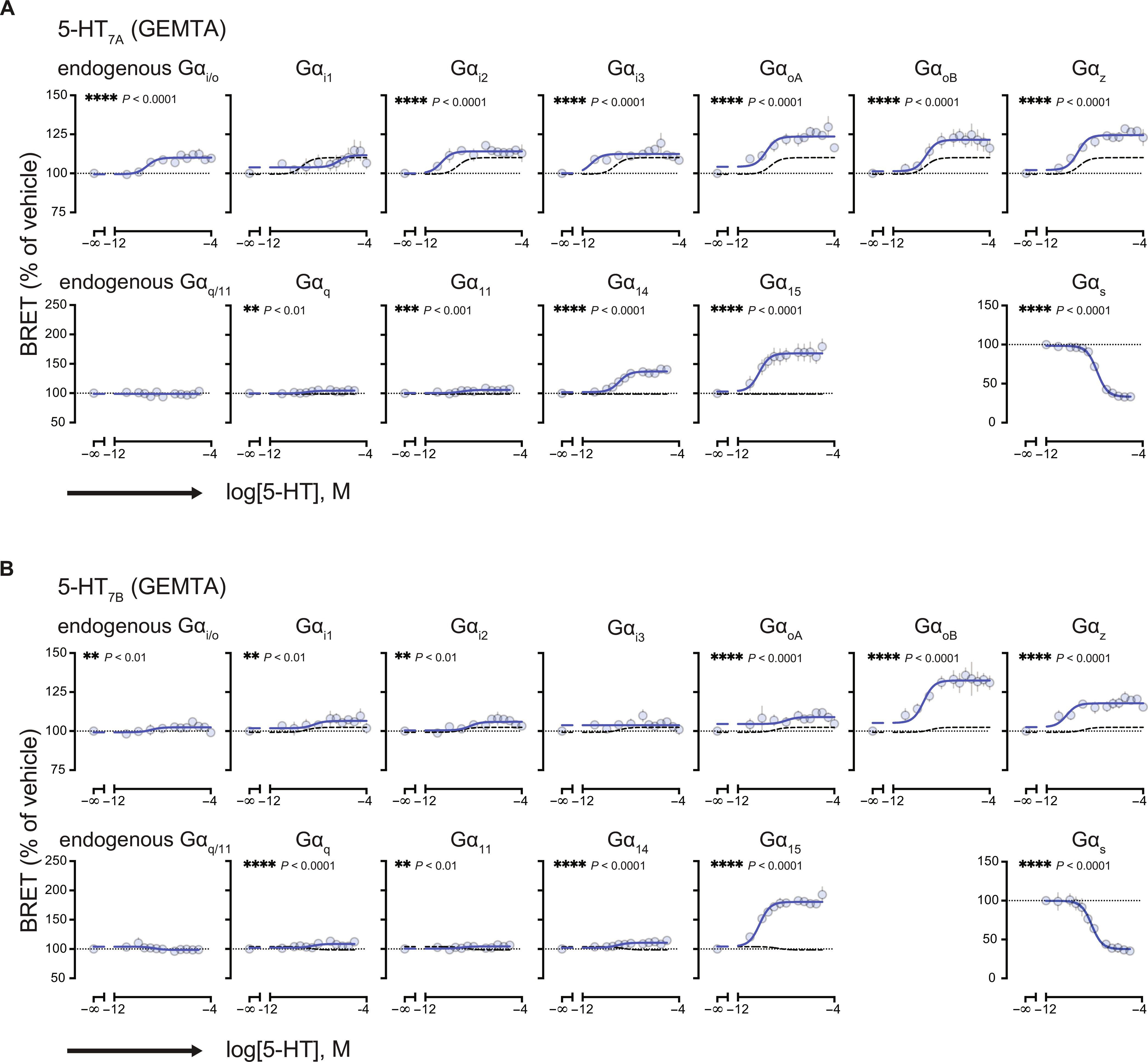
Conformation- and activation-based BRET sensors differentially report on GPCR–G protein coupling
G protein–coupled receptors (GPCRs) regulate cellular signaling processes by coupling to diverse combinations of heterotrimeric G proteins composed of Gα, Gβ, and Gγ subunits. Biosensors based on bioluminescence resonance energy transfer (BRET) have advanced our understanding of GPCR functional selectivity. Some BRET biosensors monitor ligand-induced conformational changes in the receptor or G proteins, whereas others monitor the recruitment of downstream effectors to sites of G protein activation. Here, we compared the ability of conformation-and activation-based BRET biosensors to assess the coupling of various class A and B GPCRs to specific Gα proteins in cultured cells. These GPCRs included serotonin 5-HT2A and 5-HT7 receptors, the GLP-1 receptor (GLP-1R), and the M3 muscarinic receptor. We observed different signaling profiles between the two types of sensors, highlighting how data interpretation could be affected by the nature of the biosensor. We also found that the identity of the Gβγ subunits used in the assay could differentially influence the selectivity of a receptor toward Gα subtypes, emphasizing the importance of the receptor-Gβγ pairing in determining Gα coupling specificity. Last, the addition of epitope tags to the receptor could affect stoichiometry and coupling selectivity and yield artifactual findings. These results highlight the need for careful sensor selection and experimental design when probing GPCR–G protein coupling.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science Signaling
BIOCHEMISTRY & MOLECULAR BIOLOGY-CELL BIOLOGY
CiteScore
9.50
自引率
0.00%
发文量
148
审稿时长
3-8 weeks
期刊介绍:
"Science Signaling" is a reputable, peer-reviewed journal dedicated to the exploration of cell communication mechanisms, offering a comprehensive view of the intricate processes that govern cellular regulation. This journal, published weekly online by the American Association for the Advancement of Science (AAAS), is a go-to resource for the latest research in cell signaling and its various facets.
The journal's scope encompasses a broad range of topics, including the study of signaling networks, synthetic biology, systems biology, and the application of these findings in drug discovery. It also delves into the computational and modeling aspects of regulatory pathways, providing insights into how cells communicate and respond to their environment.
In addition to publishing full-length articles that report on groundbreaking research, "Science Signaling" also features reviews that synthesize current knowledge in the field, focus articles that highlight specific areas of interest, and editor-written highlights that draw attention to particularly significant studies. This mix of content ensures that the journal serves as a valuable resource for both researchers and professionals looking to stay abreast of the latest advancements in cell communication science.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


