下载PDF
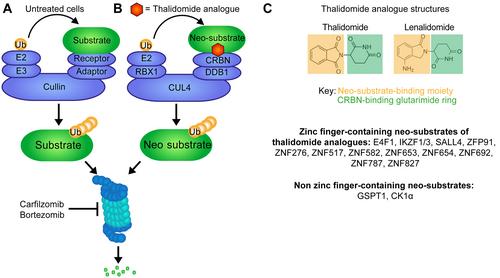
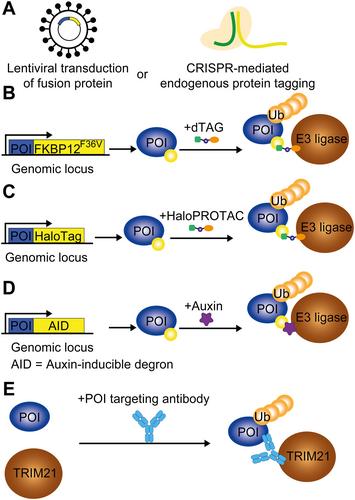
{"title":"靶向蛋白质降解在治疗和研究癌症方面的新作用。","authors":"Maximillian H Brodermann, Elizabeth K Henderson, Rob S Sellar","doi":"10.1002/path.6301","DOIUrl":null,"url":null,"abstract":"<p>The evolution of cancer treatment has provided increasingly targeted strategies both in the upfront and relapsed disease settings. Small-molecule inhibitors and immunotherapy have risen to prominence with chimeric antigen receptor T-cells, checkpoint inhibitors, kinase inhibitors, and monoclonal antibody therapies being deployed across a range of solid organ and haematological malignancies. However, novel approaches are required to target transcription factors and oncogenic fusion proteins that are central to cancer biology and have generally eluded successful drug development. Thalidomide analogues causing protein degradation have been a cornerstone of treatment in multiple myeloma, but a lack of in-depth mechanistic understanding initially limited progress in the field. When the protein cereblon (CRBN) was found to mediate thalidomide analogues' action and CRBN's neo-targets were identified, existing and novel drug development accelerated, with applications outside multiple myeloma, including non-Hodgkin's lymphoma, myelodysplastic syndrome, and acute leukaemias. Critically, transcription factors were the first canonical targets described. In addition to broadening the application of protein-degrading drugs, resistance mechanisms are being overcome and targeted protein degradation is widening the scope of druggable proteins against which existing approaches have been ineffective. Examples of targeted protein degraders include molecular glues and proteolysis targeting chimeras (PROTACs): heterobifunctional molecules that bind to proteins of interest and cause proximity-induced ubiquitination and proteasomal degradation <i>via</i> a linked E3 ligase. Twenty years since their inception, PROTACs have begun progressing through clinical trials, with early success in targeting the oestrogen receptor and androgen receptor in breast and prostate cancer respectively. This review explores important developments in targeted protein degradation to both treat and study cancer. It also considers the potential advantages and challenges in the translational aspects of developing new treatments. © 2024 The Author(s). <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"263 4-5","pages":"403-417"},"PeriodicalIF":5.6000,"publicationDate":"2024-06-17","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6301","citationCount":"0","resultStr":"{\"title\":\"The emerging role of targeted protein degradation to treat and study cancer\",\"authors\":\"Maximillian H Brodermann, Elizabeth K Henderson, Rob S Sellar\",\"doi\":\"10.1002/path.6301\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<p>The evolution of cancer treatment has provided increasingly targeted strategies both in the upfront and relapsed disease settings. Small-molecule inhibitors and immunotherapy have risen to prominence with chimeric antigen receptor T-cells, checkpoint inhibitors, kinase inhibitors, and monoclonal antibody therapies being deployed across a range of solid organ and haematological malignancies. However, novel approaches are required to target transcription factors and oncogenic fusion proteins that are central to cancer biology and have generally eluded successful drug development. Thalidomide analogues causing protein degradation have been a cornerstone of treatment in multiple myeloma, but a lack of in-depth mechanistic understanding initially limited progress in the field. When the protein cereblon (CRBN) was found to mediate thalidomide analogues' action and CRBN's neo-targets were identified, existing and novel drug development accelerated, with applications outside multiple myeloma, including non-Hodgkin's lymphoma, myelodysplastic syndrome, and acute leukaemias. Critically, transcription factors were the first canonical targets described. In addition to broadening the application of protein-degrading drugs, resistance mechanisms are being overcome and targeted protein degradation is widening the scope of druggable proteins against which existing approaches have been ineffective. Examples of targeted protein degraders include molecular glues and proteolysis targeting chimeras (PROTACs): heterobifunctional molecules that bind to proteins of interest and cause proximity-induced ubiquitination and proteasomal degradation <i>via</i> a linked E3 ligase. Twenty years since their inception, PROTACs have begun progressing through clinical trials, with early success in targeting the oestrogen receptor and androgen receptor in breast and prostate cancer respectively. This review explores important developments in targeted protein degradation to both treat and study cancer. It also considers the potential advantages and challenges in the translational aspects of developing new treatments. © 2024 The Author(s). <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>\",\"PeriodicalId\":232,\"journal\":{\"name\":\"The Journal of Pathology\",\"volume\":\"263 4-5\",\"pages\":\"403-417\"},\"PeriodicalIF\":5.6000,\"publicationDate\":\"2024-06-17\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6301\",\"citationCount\":\"0\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"The Journal of Pathology\",\"FirstCategoryId\":\"3\",\"ListUrlMain\":\"https://onlinelibrary.wiley.com/doi/10.1002/path.6301\",\"RegionNum\":2,\"RegionCategory\":\"医学\",\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"Q1\",\"JCRName\":\"ONCOLOGY\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/path.6301","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


