控制质子化螺环 4H 吡唑的 1,5-sigmatropic 漂移
IF 1.9
4区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
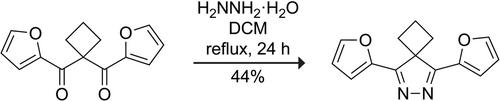
1,3-二酮与肼缩合生成 4H-吡唑是一条经过 4H-吡唑-1-鎓中间体的成熟合成路线。在通向含有环丁烷螺环的 3,5-二苯基-4H-吡唑的路线中,密度泛函理论计算预测和实验表明,质子化的中间体会发生快速的 1,5-sigmatropic 转变,形成四氢环戊并[c]吡唑。用 2-呋喃基取代 3,5-二苯基后,1,5-移位的计算速率降低了 6.2 × 105 倍,从而能够分离出新的螺环 4H 吡唑,用于点击化学。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Taming the 1,5-sigmatropic shift across protonated spirocyclic 4H-pyrazoles
The condensation of 1,3-diketones with hydrazine to access 4H-pyrazoles is a well-established synthetic route that travels through a 4H-pyrazol-1-ium intermediate. In the route to a 3,5-diphenyl-4H-pyrazole containing a cyclobutane spirocycle, density functional theory calculations predict, and experiments show that the protonated intermediate undergoes a rapid 1,5-sigmatropic shift to form a tetrahydrocyclopenta[c]pyrazole. Replacing the 3,5-diphenyl groups with 2-furanyl groups decreases the calculated rate of the 1,5-sigmatropic shift by 6.2 × 105-fold and enables the isolation of new spirocyclic 4H-pyrazoles for click chemistry.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
3.60
自引率
11.10%
发文量
161
审稿时长
2.3 months
期刊介绍:
The Journal of Physical Organic Chemistry is the foremost international journal devoted to the relationship between molecular structure and chemical reactivity in organic systems. It publishes Research Articles, Reviews and Mini Reviews based on research striving to understand the principles governing chemical structures in relation to activity and transformation with physical and mathematical rigor, using results derived from experimental and computational methods. Physical Organic Chemistry is a central and fundamental field with multiple applications in fields such as molecular recognition, supramolecular chemistry, catalysis, photochemistry, biological and material sciences, nanotechnology and surface science.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


