钆镉相图的热力学描述
IF 1.7
4区 材料科学
Q4 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
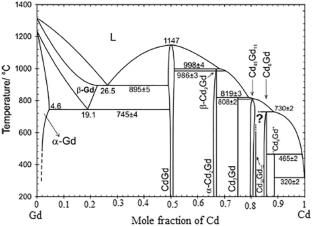
在 CALPHAD(CALculation of PHAse Diagram)方法的帮助下,对 Gd-Cd 二元体系进行了热力学优化。GdCd3、Gd13Cd58 和 GdCd8 被视为化学计量化合物,而溶液模型则用于描述液相、HCP_A3(α Gd)相、BCC_A2(β Gd)相和 HCP_A3(Cd)相。金属间化合物 GdCd、α-GdCd2、β-GdCd2、Gd11Cd45 和 GdCd6 具有同质性范围,采用双子晶格模型进行处理。基于热力学模型的计算结果与相图数据和实验热力学值十分吻合。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Thermodynamic Description of the Gadolinium-Cadmium Phase Diagram
The thermodynamic optimization of the Gd-Cd binary system was carried out with the help of CALPHAD (CALculation of PHAse Diagram) method., GdCd3, Gd13Cd58 and GdCd8 have been treated as stoichiometric compounds while a solution model has been used for the description of the liquid, HCP_A3 (αGd), BCC_A2 (βGd) and HCP_A3 (Cd) phases. The intermetallic compounds GdCd,α-GdCd2, β-GdCd2, Gd11Cd45 and GdCd6, which have homogeneity ranges, were treated by a two-sublattice model. The calculations based on the thermodynamic modeling are in a good agreement with the phase diagram data and experimental thermodynamic values.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Phase Equilibria and Diffusion
工程技术-材料科学:综合
CiteScore
2.50
自引率
7.10%
发文量
70
审稿时长
1 months
期刊介绍:
The most trusted journal for phase equilibria and thermodynamic research, ASM International''s Journal of Phase Equilibria and Diffusion features critical phase diagram evaluations on scientifically and industrially important alloy systems, authored by international experts.
The Journal of Phase Equilibria and Diffusion is critically reviewed and contains basic and applied research results, a survey of current literature and other pertinent articles. The journal covers the significance of diagrams as well as new research techniques, equipment, data evaluation, nomenclature, presentation and other aspects of phase diagram preparation and use.
Content includes information on phenomena such as kinetic control of equilibrium, coherency effects, impurity effects, and thermodynamic and crystallographic characteristics. The journal updates systems previously published in the Bulletin of Alloy Phase Diagrams as new data are discovered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


