数字医疗技术需要监管和报销,以实现灵活的互动和分组
IF 12.4
1区 医学
Q1 HEALTH CARE SCIENCES & SERVICES
引用次数: 0
摘要
数字健康技术(DHT)在医学领域的应用范围越来越广。我们描述了一种新出现的现象,即把具有临床用例并获得监管部门批准的单个 DHT 组合成套件,以便在规定的环境中执行特定的临床任务。分组实例包括用于远程监控或智能诊所的成套设备。本文是两篇系列文章中的第一篇,我们将介绍这些成套设备和相关新型护理路径在实施过程中遇到的挑战以及在监管、卫生技术评估和报销框架方面存在的局限性。本文章由计算机程序翻译,如有差异,请以英文原文为准。

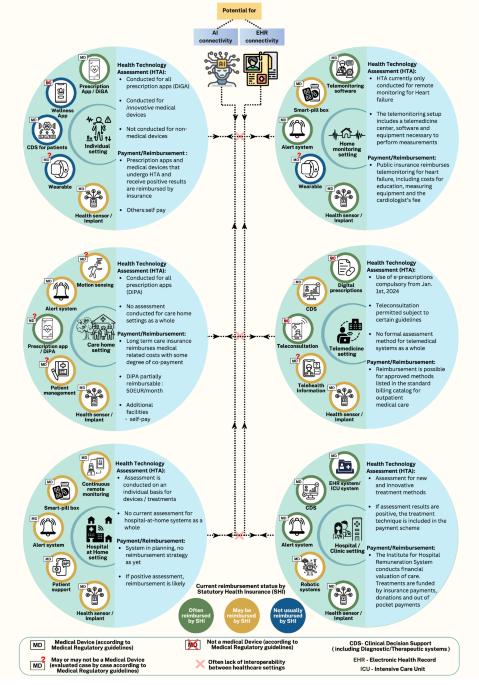
Digital health technologies need regulation and reimbursement that enable flexible interactions and groupings
Digital Health Technologies (DHTs) are being applied in a widening range of scenarios in medicine. We describe the emerging phenomenon of the grouping of individual DHTs, with a clinical use case and regulatory approval in their own right, into packages to perform specific clinical tasks in defined settings. Example groupings include suites of devices for remote monitoring, or for smart clinics. In this first article of a two-article series, we describe challenges in implementation and limitations in frameworks for the regulation, health technology assessment, and reimbursement of these device suites and linked novel care pathways.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

NPJ Digital Medicine
Multiple-
CiteScore
25.10
自引率
3.30%
发文量
170
审稿时长
15 weeks
期刊介绍:
npj Digital Medicine is an online open-access journal that focuses on publishing peer-reviewed research in the field of digital medicine. The journal covers various aspects of digital medicine, including the application and implementation of digital and mobile technologies in clinical settings, virtual healthcare, and the use of artificial intelligence and informatics.
The primary goal of the journal is to support innovation and the advancement of healthcare through the integration of new digital and mobile technologies. When determining if a manuscript is suitable for publication, the journal considers four important criteria: novelty, clinical relevance, scientific rigor, and digital innovation.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


