破解范可尼贫血症蛋白翻译后修饰的作用及其对肿瘤发生的影响。
IF 4.8
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
摘要
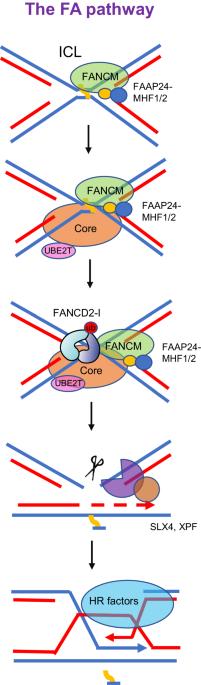
范可尼贫血症(FA)是一种常染色体或 X 连锁人类疾病,以骨髓衰竭、癌症易感性和各种发育异常为特征。迄今为止,已发现至少 22 个 FA 基因(FANCA-W)。任何一个 FA 基因的种系失活都会导致 FA 症状。FA 基因编码的蛋白质参与了范可尼贫血症通路,该通路因其在 DNA 链间交联(ICLs)修复中的作用而闻名。此外,也有报道称它在复制压力下维护基因组的作用。FA 蛋白的翻译后修饰(PTMs),尤其是磷酸化和泛素化,成为在 ICL 修复或复制应激反应过程中激活 FA 通路的关键决定因素。FA 通路的失活导致染色体不稳定性增加,从而构成了一种遗传易感性,有利于癌症的易感性和肿瘤发生的加剧。在这篇综述中,我们结合最近对 FA 蛋白的结构分析,总结了不同 PTMs 在调控 FA 通路中的功能,并讨论了 PTMs 突变对肿瘤发生和发展的潜在贡献。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Deciphering the role of post-translational modifications in fanconi anemia proteins and their influence on tumorigenesis
Fanconi anemia (FA) is an autosomal or X-linked human disease, characterized by bone marrow failure, cancer susceptibility and various developmental abnormalities. So far, at least 22 FA genes (FANCA-W) have been identified. Germline inactivation of any one of these FA genes causes FA symptoms. Proteins encoded by FA genes are involved in the Fanconi anemia pathway, which is known for its roles in DNA inter-strand crosslinks (ICLs) repair. Besides, its roles in genome maintenance upon replication stress has also been reported. Post-translational modifications (PTMs) of FA proteins, particularly phosphorylation and ubiquitination, emerge as critical determinants in the activation of the FA pathway during ICL repair or replication stress response. Consequent inactivation of the FA pathway engenders heightened chromosomal instability, thereby constituting a genetic susceptibility conducive to cancer predisposition and the exacerbation of tumorigenesis. In this review, we have combined recent structural analysis of FA proteins and summarized knowledge on the functions of different PTMs in regulating FA pathways, and discuss potential contributions stemming from mutations at PTMs to the genesis and progression of tumorigenesis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


