LKB1 和 KEAP1 的同时缺失可增强 KRAS 突变肺癌中 SHMT 介导的抗氧化防御能力
IF 20.8
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
摘要
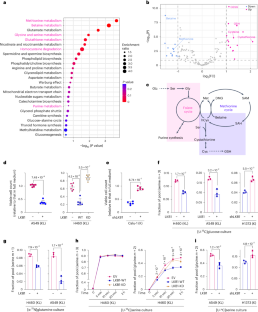
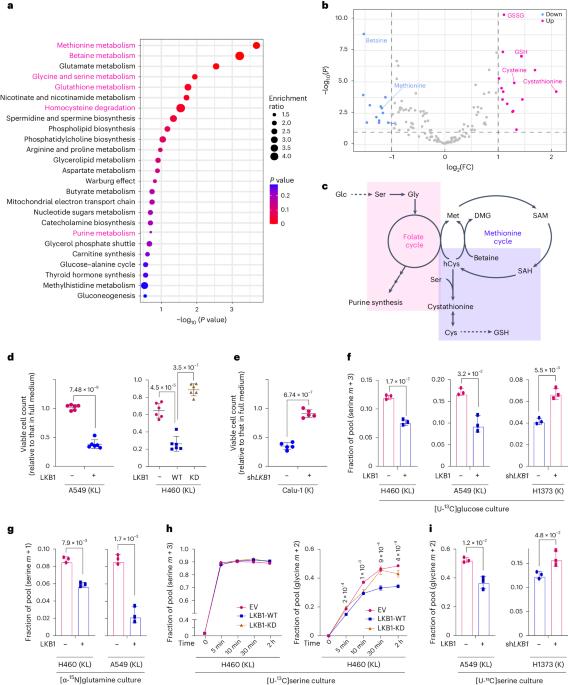
KRAS 和肿瘤抑制因子 LKB1(KL NSCLC)同时发生突变的非小细胞肺癌(NSCLC)对大多数疗法都是难治性的,是预测结果最差的癌症之一。在这里,我们描述了与丝氨酸-甘氨酸-一碳(SGOC)代谢相关的KL诱导的代谢脆弱性。利用来自人类 NSCLC 的 RNA-seq 和代谢组学数据,我们发现 LKB1 的缺失通过丝氨酸羟甲基转移酶(SHMT)增强了 SGOC 的代谢。LKB1 的缺失与 KEAP1 的缺失共同作用,通过盐诱导激酶(SIK)-NRF2 轴的失活激活了 SHMT,并满足了抗氧化防御对一碳单位的更高需求。化学和遗传的 SHMT 抑制增加了细胞对氧化应激和细胞死亡的敏感性。此外,在KEAP1突变的KL肿瘤中,SHMT抑制剂增强了紫杉醇(诱导氧化应激的NSCLC一线疗法)的体内疗效。这些数据揭示了这种侵袭性极强的 NSCLC 分子亚型是如何满足其代谢要求的,并为治疗策略提供了启示。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Concurrent loss of LKB1 and KEAP1 enhances SHMT-mediated antioxidant defence in KRAS-mutant lung cancer
Non-small-cell lung cancer (NSCLC) with concurrent mutations in KRAS and the tumour suppressor LKB1 (KL NSCLC) is refractory to most therapies and has one of the worst predicted outcomes. Here we describe a KL-induced metabolic vulnerability associated with serine–glycine-one-carbon (SGOC) metabolism. Using RNA-seq and metabolomics data from human NSCLC, we uncovered that LKB1 loss enhanced SGOC metabolism via serine hydroxymethyltransferase (SHMT). LKB1 loss, in collaboration with KEAP1 loss, activated SHMT through inactivation of the salt-induced kinase (SIK)–NRF2 axis and satisfied the increased demand for one-carbon units necessary for antioxidant defence. Chemical and genetic SHMT suppression increased cellular sensitivity to oxidative stress and cell death. Further, the SHMT inhibitor enhanced the in vivo therapeutic efficacy of paclitaxel (first-line NSCLC therapy inducing oxidative stress) in KEAP1-mutant KL tumours. The data reveal how this highly aggressive molecular subtype of NSCLC fulfills their metabolic requirements and provides insight into therapeutic strategies. Lee et al. identify SHMT and one-carbon metabolism as a metabolic vulnerability conferred by LKB1 and KEAP1 loss in KRAS-mutant lung cancer.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


