GIPR 遗传变异的特征揭示了 β-arrestin 对代谢表型的贡献
IF 18.9
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
摘要
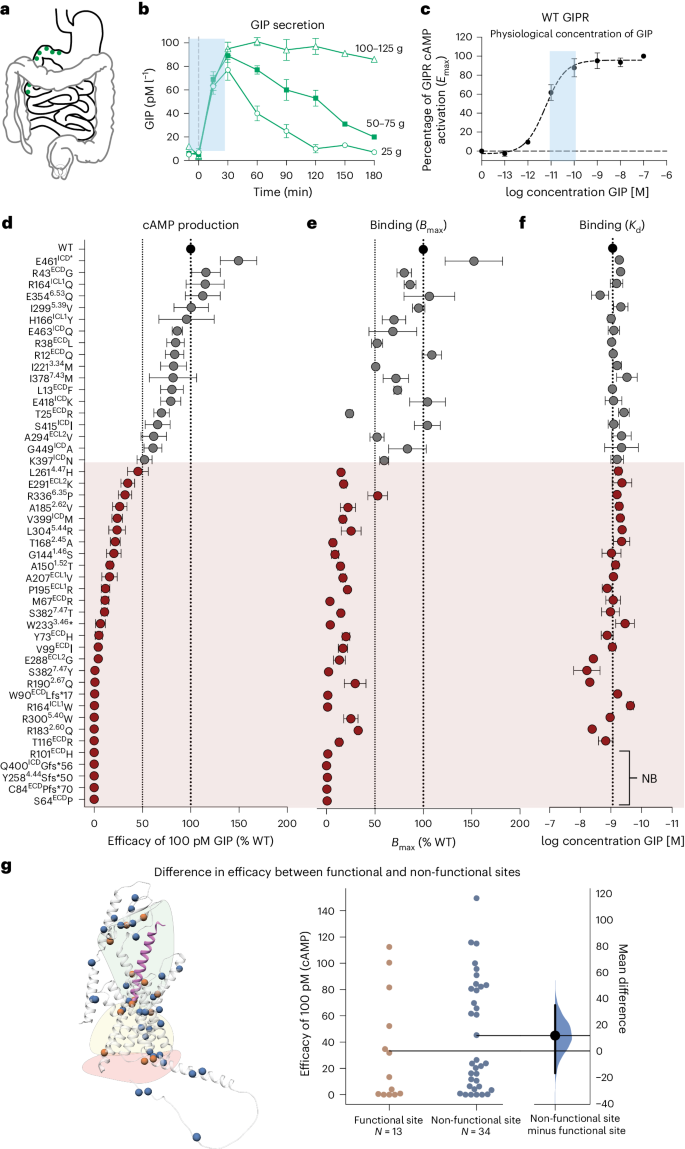
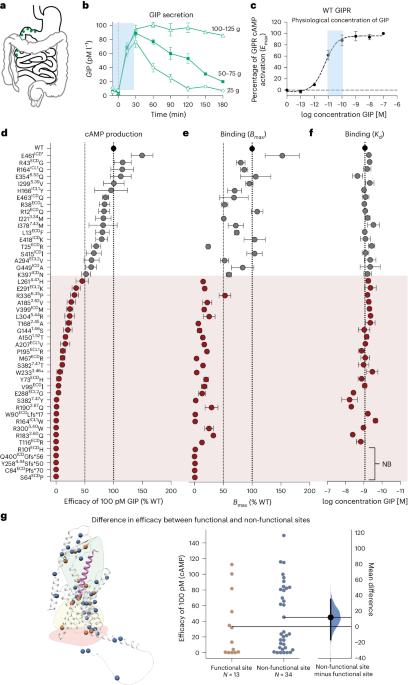
基于胰岛素的疗法在防治肥胖症和 2 型糖尿病方面非常成功1。然而,葡萄糖依赖性胰岛素多肽(GIP)受体(GIPR)的激活和抑制与胰高血糖素样肽-1(GLP-1)受体(GLP-1R)的激活相结合,却产生了相似的临床效果,GIPR-GLP-1R联合激动剂替扎帕肽(tirzepatide)2 和结合GIPR拮抗与GLP-1R激动的AMG-133(参考文献3)就证明了这一点。这强调了更好地了解 GIP 系统的重要性。在这里,我们通过对 47 种 GIPR 变体的体外药理学特征描述、临床表型的负荷测试和体内研究相结合,证明了 GIPR 功能对 β-阻遏素招募的必要性。对具有不同配体结合能力、Gs活化(环磷酸腺苷产生)和β-阿restin 2募集和内化的变体进行的负担测试表明,与仅在Gs信号方面受损的变体不同,在Gs和β-阿restin 2募集方面均受损的变体会导致脂肪相关特征降低。变体的内质体 Gs 介导的信号传导显示出对 β-arrestin 的依赖性,β-arrestin 2 的遗传消减会影响环磷酸腺苷的产生,并降低 GIP 对雄性小鼠血糖控制的功效。这项研究强调了β-阿司匹林在调节GIPR信号传导和整体保持生物活性方面的重要影响,这可能会促进GIPR系统靶向治疗的新发展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Characterization of genetic variants of GIPR reveals a contribution of β-arrestin to metabolic phenotypes
Incretin-based therapies are highly successful in combatting obesity and type 2 diabetes1. Yet both activation and inhibition of the glucose-dependent insulinotropic polypeptide (GIP) receptor (GIPR) in combination with glucagon-like peptide-1 (GLP-1) receptor (GLP-1R) activation have resulted in similar clinical outcomes, as demonstrated by the GIPR–GLP-1R co-agonist tirzepatide2 and AMG-133 (ref. 3) combining GIPR antagonism with GLP-1R agonism. This underlines the importance of a better understanding of the GIP system. Here we show the necessity of β-arrestin recruitment for GIPR function, by combining in vitro pharmacological characterization of 47 GIPR variants with burden testing of clinical phenotypes and in vivo studies. Burden testing of variants with distinct ligand-binding capacity, Gs activation (cyclic adenosine monophosphate production) and β-arrestin 2 recruitment and internalization shows that unlike variants solely impaired in Gs signalling, variants impaired in both Gs and β-arrestin 2 recruitment contribute to lower adiposity-related traits. Endosomal Gs-mediated signalling of the variants shows a β-arrestin dependency and genetic ablation of β-arrestin 2 impairs cyclic adenosine monophosphate production and decreases GIP efficacy on glucose control in male mice. This study highlights a crucial impact of β-arrestins in regulating GIPR signalling and overall preservation of biological activity that may facilitate new developments in therapeutic targeting of the GIPR system. Molecular pharmacological characterization and association testing of human GIPR genetic variants with follow-up analysis in mice shows that β-arrestins regulate GIPR signalling and thereby strongly contribute to metabolic outcomes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


