tRNA Gm18甲基转移酶TARBP1通过谷氨酰胺的代谢重编程促进肝细胞癌的进展。
IF 13.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
癌细胞依靠新陈代谢重编程来维持快速生长和增殖所需的大量能量。谷氨酰胺代谢在癌症中经常出现失调,目前正被用作潜在的治疗靶点。通过 CRISPR/Cas9 干扰(CRISPRi)筛选,我们发现 TARBP1(TAR(HIV-1)RNA 结合蛋白 1)是参与癌细胞谷氨酰胺依赖的关键调控因子。与这一发现相一致的是,TARBP1 的扩增和过表达经常在各种癌症中被观察到。敲除 TARBP1 能显著抑制细胞增殖、集落形成和异种移植肿瘤的生长。从机理上讲,TARBP1可选择性地甲基化和稳定一小部分tRNA,从而促进谷氨酰胺转运体-ASCT2(又称SLC1A5)蛋白质的高效合成和谷氨酰胺的输入,为癌细胞的生长提供动力。此外,我们还发现,在临床队列中,TARBP1 和 ASCT2 的基因表达同时上调,而它们的上调与 HCC(肝细胞癌)的不良预后相关。综上所述,本研究揭示了 TARBP1 在 HCC 进展过程中协调 tRNA 可用性和谷氨酰胺摄取的意外作用,并为肿瘤治疗提供了潜在靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
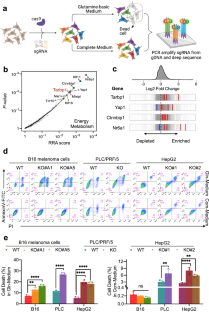
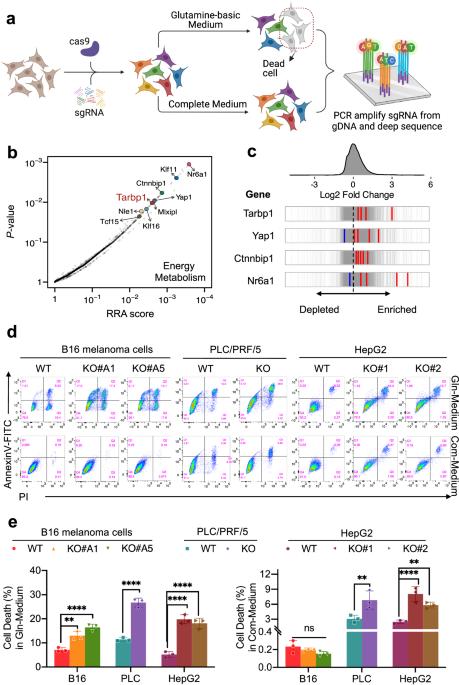
The tRNA Gm18 methyltransferase TARBP1 promotes hepatocellular carcinoma progression via metabolic reprogramming of glutamine
Cancer cells rely on metabolic reprogramming to sustain the prodigious energetic requirements for rapid growth and proliferation. Glutamine metabolism is frequently dysregulated in cancers and is being exploited as a potential therapeutic target. Using CRISPR/Cas9 interference (CRISPRi) screening, we identified TARBP1 (TAR (HIV-1) RNA Binding Protein 1) as a critical regulator involved in glutamine reliance of cancer cell. Consistent with this discovery, TARBP1 amplification and overexpression are frequently observed in various cancers. Knockout of TARBP1 significantly suppresses cell proliferation, colony formation and xenograft tumor growth. Mechanistically, TARBP1 selectively methylates and stabilizes a small subset of tRNAs, which promotes efficient protein synthesis of glutamine transporter-ASCT2 (also known as SLC1A5) and glutamine import to fuel the growth of cancer cell. Moreover, we found that the gene expression of TARBP1 and ASCT2 are upregulated in combination in clinical cohorts and their upregulation is associated with unfavorable prognosis of HCC (hepatocellular carcinoma). Taken together, this study reveals the unexpected role of TARBP1 in coordinating the tRNA availability and glutamine uptake during HCC progression and provides a potential target for tumor therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell Death and Differentiation
生物-生化与分子生物学
CiteScore
24.70
自引率
1.60%
发文量
181
审稿时长
3 months
期刊介绍:
Mission, vision and values of Cell Death & Differentiation:
To devote itself to scientific excellence in the field of cell biology, molecular biology, and biochemistry of cell death and disease.
To provide a unified forum for scientists and clinical researchers
It is committed to the rapid publication of high quality original papers relating to these subjects, together with topical, usually solicited, reviews, meeting reports, editorial correspondence and occasional commentaries on controversial and scientifically informative issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


