Caspase-Activated DNase(Caspase-Activated DNase)可驱动炎症,有助于抵御病毒感染。
IF 13.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
线粒体通过凋亡途径中的亚致死信号对感染做出反应。线粒体信号可能具有炎症性,但人们对其机制仅有部分了解。我们的研究表明,Caspase-activated DNase(CAD)的激活介导了线粒体的促炎功能,并在很大程度上促进了宿主抵御病毒感染的能力。在缺乏 CAD 的细胞中,亚致死信号的促炎活性降低。实验性激活CAD会引起短暂的DNA损伤和明显的DNA损伤反应,涉及主要的激酶信号通路、NF-κB和cGAS/STING,驱动干扰素、细胞因子/凝血因子的产生并吸引中性粒细胞。CAD激活后的转录反应与微生物感染后的反应相似。CAD缺陷细胞对病毒感染的反应减弱。感染流感病毒的 CAD 缺陷小鼠肺组织炎症减轻,病毒滴度升高,体重减轻。因此,CAD 将线粒体凋亡系统和细胞死亡 caspases 与宿主防御联系起来。CAD 驱动的 DNA 损伤是感染炎症反应的一个生理要素。本文章由计算机程序翻译,如有差异,请以英文原文为准。
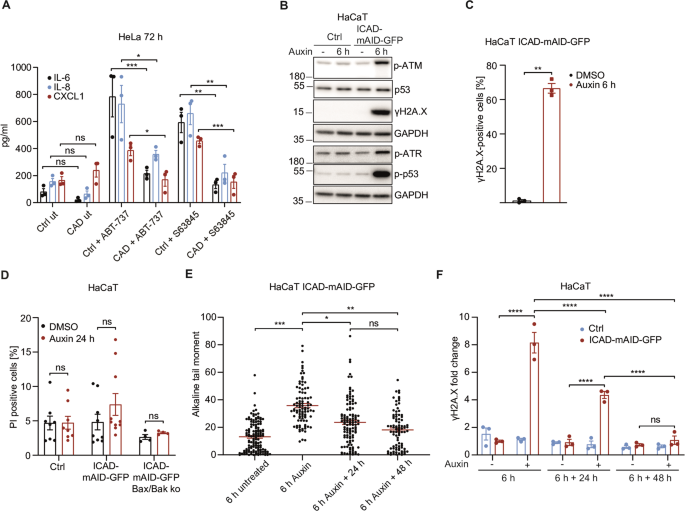
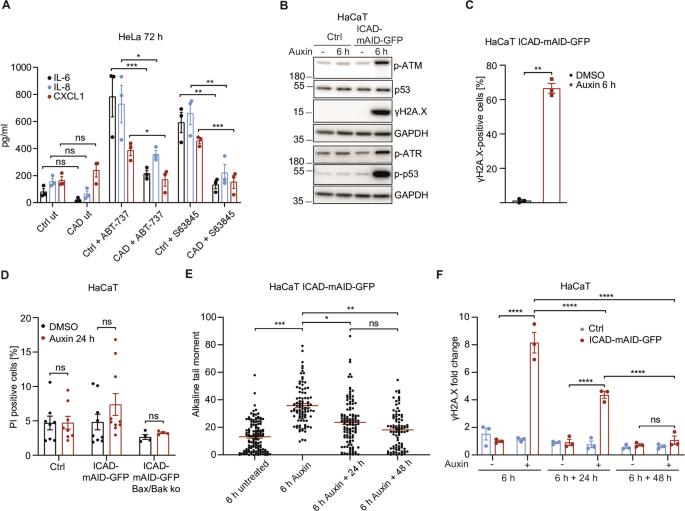
The Caspase-Activated DNase drives inflammation and contributes to defense against viral infection
Mitochondria react to infection with sub-lethal signals in the apoptosis pathway. Mitochondrial signals can be inflammatory but mechanisms are only partially understood. We show that activation of the caspase-activated DNase (CAD) mediates mitochondrial pro-inflammatory functions and substantially contributes to host defense against viral infection. In cells lacking CAD, the pro-inflammatory activity of sub-lethal signals was reduced. Experimental activation of CAD caused transient DNA-damage and a pronounced DNA damage response, involving major kinase signaling pathways, NF-κB and cGAS/STING, driving the production of interferon, cytokines/chemokines and attracting neutrophils. The transcriptional response to CAD-activation was reminiscent of the reaction to microbial infection. CAD-deficient cells had a diminished response to viral infection. Influenza virus infected CAD-deficient mice displayed reduced inflammation in lung tissue, higher viral titers and increased weight loss. Thus, CAD links the mitochondrial apoptosis system and cell death caspases to host defense. CAD-driven DNA damage is a physiological element of the inflammatory response to infection.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell Death and Differentiation
生物-生化与分子生物学
CiteScore
24.70
自引率
1.60%
发文量
181
审稿时长
3 months
期刊介绍:
Mission, vision and values of Cell Death & Differentiation:
To devote itself to scientific excellence in the field of cell biology, molecular biology, and biochemistry of cell death and disease.
To provide a unified forum for scientists and clinical researchers
It is committed to the rapid publication of high quality original papers relating to these subjects, together with topical, usually solicited, reviews, meeting reports, editorial correspondence and occasional commentaries on controversial and scientifically informative issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


