蛋白质折叠的爆发期中间体内部。疏水基团的水合作用。
IF 3.3
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
我们使用滴定量热计研究了 "爆发相 "折叠中间体形成的热效应。结果表明,与原生结构形成的总热效应不同,它既可以是正的,也可以是负的,这取决于温度。分析了这种矛盾行为的原因。得出了非极性基团脱水在折叠第一阶段起主导作用的结论。本文章由计算机程序翻译,如有差异,请以英文原文为准。
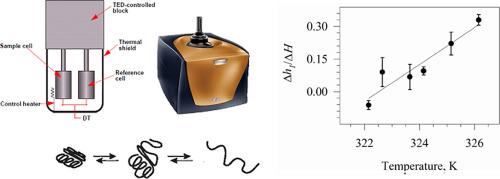
Inside of the burst-phase intermediate of a protein folding. Hydration of hydrophobic groups
The thermal effect of the formation of the “burst-phase” folding intermediate has been studied using a titration calorimeter. It is shown that, unlike the total thermal effect of native structure formation, it can be both positive and negative depending on the temperature. The reasons for this paradoxical behavior are analyzed. A conclusion is drawn about the leading role of dehydration of non-polar groups in the first stage of folding.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
Biophysical chemistry
生物-生化与分子生物学
CiteScore
6.10
自引率
10.50%
发文量
121
审稿时长
20 days
期刊介绍:
Biophysical Chemistry publishes original work and reviews in the areas of chemistry and physics directly impacting biological phenomena. Quantitative analysis of the properties of biological macromolecules, biologically active molecules, macromolecular assemblies and cell components in terms of kinetics, thermodynamics, spatio-temporal organization, NMR and X-ray structural biology, as well as single-molecule detection represent a major focus of the journal. Theoretical and computational treatments of biomacromolecular systems, macromolecular interactions, regulatory control and systems biology are also of interest to the journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


